Commentary :
This
article introduces the characteristics of postprandial Very Low Density Lipoprotein (VLDL)
remnants (remnant lipoproteins; RLP) in plasma which significantly increased
after fat load as a major component of increased Triglycerides (TG) and
involved in obesity and insulin resistance. It has been long believed that
postprandial RLP, mainly Chylomicron
(CM) remnants, increases as the result of disturbed lipoprotein lipase (LPL)
activity caused by insulin resistance, etc. However, based on this report, we recently
proposed that elevated postprandial VLDL remnants produced by food intake, such
as excessive fat and fructose, cause obesity and insulin resistance when
exposed continuously [1]. VLDL remnants, but not CM remnants, is the key word
of this article and VLDL remnants play a definitive role as a bridge between
food intake and its metabolism. Here, we have explained the bridging role of
VLDL remnants between the habit of food intake and its metabolism in body.
Following 6 aspects between fat-rich meal intake and the increase of plasma
postprandial TG and RLP are explained. (1) Why TG and RLP increase after food
intake? (2) Which lipoproteins increase most after food intake? (3) What
percentage of increased TG after food is comprised of RLP-TG? (4) How the
increased TG is metabolized by LPL? (5) The increase of postprandial RLP is the
result of obesity and insulin resistance or cause of obesity and insulin
resistance? (6)Why postprandial TG is a risk of cardiovascular diseases?
Dietary fat provides
as much as 30% to 40% of total daily caloric intake in the western diet and TG
constitutes the majority of that fat. Dietary
Long-Chain Triglycerides (LCTs), the most common dietary lipid structures, are
mainly digested into two fatty acids and an SN-2 monoglyceride molecule [2].
Re-esterification of fatty acids occurs in the enterocytes of the small
intestine. Subsequently, the resulting LCTs are incorporated into CM particles
and released into the blood through the lymphatic system after
food intake. Therefore, plasma TG concentration increases significantly after
food intake, especially after fat-rich meal with LCTs [3]. But certain fatty
acids such as Medium Chain Triglycerides (MCT) and Diacylglycerol (DG) do not
increase plasma TG, because MCT and DG are absorbed directly into the portal
circulation to liver rather than being incorporated into chylomicron particles
at intestine [4,5]. Therefore, fat intake (LCTs) affects the increase of CM
formation and secrete into blood circulation with increased amount of TG mostly
as VLDL remnants in 3-6 hours after fat intake. Zilversmit
first proposed the postprandial increase of TG to be the most common form of hyperlipidemia which
associated with increased RLP as a risk for Cardiovascular Disease (CVD).
Therefore, the postprandial TG increase has been long believed as the increase
of TG in CM, CM remnants in plasma [3]. Because,
increase of RLP and its ratio in the postprandial TG has not been clearly shown
by ultracentrifugation separation (IDL) or other separation methods [6]. Using
RLP immuno-separation method, the differences between increased TG and RLP in
the fasting and postprandial plasma have been clarified [7-11]. Although the
cholesterol content in RLP (RLP-C) is commonly found to be less than 10% even
in the postprandial plasma TC, TG content in RLP (RLP-TG) is found to be more
than 20% in the fasting plasma TG and as much as 50% in the postprandial plasma
TG under various physiological conditions [12]. The postprandial RLP contained
both apoB-48 and apoB-100 carrying particles. The increase of RLP apoB-100
particles (VLDL remnants) in fact was much greater (more than 80%) than that of
apoB-48 containing lipoproteins (CM remnants) in the postprandial state [13-15].
Because the particle sizes of postprandial RLP-apoB48 and RLP-apoB100 are very
similar (postprandial apoB48 particles in plasma are not large as being
believed) [10], we found that major component of postprandial TG increased is
VLDL remnants, but not CM remnants. Possibly, most of CM and CM remnants
increased in plasma after food intake are incorporated into liver within a very
short time [16,17] and re-constituted to VLDL and secreted as VLDL remnants in
plasma. Significantly
higher RLP-TG is contained in the postprandial plasma than in the fasting
plasma when the TG level is adjusted as the RLP-TG/TG ratio [18]. These results
show that the amount and ratio of RLP in the postprandial TG increased
significantly compared with the fasting plasma TG. In particular, the increase
in the postprandial delta RLP-TG (postprandial RLP-TG minus fasting RLP-TG)
levels contributed to approximately 50-60% of the increase in the postprandial
delta TG (postprandial TG minus fasting TG) after regular meal intake. However,
more than 80% of the increased delta TG was comprised of delta RLP-TG after a
fat load or fat rich meal [18]. These results clearly show that the kind of
food as contained in a fat rich meal greatly enhance the formation of RLP in
the postprandial plasma compared with a regular meal. Marcoux et al. [19], Ooi
et al. [20] and Nakajima et al. [21] previously reported similar results in
small number of Caucasian and Japanese volunteers, in whom approximately 60-80%
of the delta RLP-TG in delta TGs were found in 3-6 h after a fat rich meal. The
rest of the increased TG consisted of increased non-RLP-VLDL-TG, LDL-TG and
HDL-TG in the postprandial plasma, but do not comprise of TG a much as RLP-TG. We
have found that majority of LPL in plasma is bound to RLP and released into
circulation as RLP-LPL complex both in pre-heparin and post-heparin plasma [22].
LPL bound to RLP showed no activity in non-heparin plasma and didnt increase
after fat load in spite of the increase of RLP [23]. However, LPL levels in
non-heparin plasma reflect the LPL activity for hydrolysis of CM and VLDL at
endothelium. RLP-TG concentration and particle size increased in plasma after
food intake is mainly regulated by LPL activity at endothelium together with
other factors such as GPIHBP1 [24] and apoA5 [25]. A significant increase in the
RLP-TG/RLP-C ratio was always higher in the postprandial plasma and ratio of
LPL/RLP-TG was significantly lower. When LPL activity is not sufficient to
hydrolyze overloaded CM or VLDL on the endothelial cells, less efficient
hydrolysis occur and enhance the formation of less metabolized, large RLP
particles along with the higher RLP-TG/RLP-C ratio. Those RLP particles carry a
significantly lower LPL compared to small RLP particles, as shown by the low
LPL/RLP-TG ratio [23, 26, 27]. Therefore, when the LPL activity and
concentration is low, overloaded CM and VLDL are not hydrolyzed enough. Also
when CM and VLDL are over loaded, LPL cant hydrolyze the excessive amount of
TG-rich lipoproteins, resulting large size RLP particles are secreted into the
postprandial plasma. As large RLP particles carry small amount of LPL, the
function as ligand for the receptor incorporation of remnants [28], may become
less effective for the clearance of remnants and accumulate more in plasma [22].
These results suggest that the large RLP with reduced ratio of bound LPL
(LPL/RLP-TG) found in the postprandial plasma is a higher risk factor for obesity, insulin resistance and
cardiovascular disease, as shown previous reported [29-31]. We
have long thought that postprandial remnant lipoproteins (RLP) in plasma are
significantly increased as the results of disturbed lipoprotein metabolism
followed by the obesity and insulin resistance. Thereby, we believed that the
insulin resistance as the result of obesity caused the enhancement of
postprandial RLP formation. However on the contrary, we have proposed that RLP
cause to induce the insulin resistance as the results of obesity which is
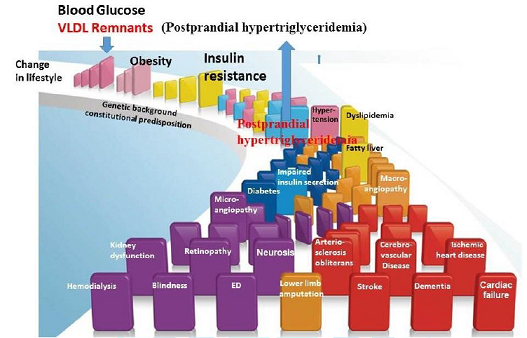
induced by the excessive supply of RLP to visceral fat. Since the increase of
VLDL remnants in plasma is the first step of lipid metabolism right after
fat-rich meal intake as blood sugar after carbohydrate intake, we proposed that
postprandial VLDL remnants are the other factor which enables to play the role
for the storage of TG in adiposetissue from the circulation. The consumption of fat-rich meal and fructose
are known to increase postprandial TG, fasting and postprandial RLP-C and
RLP-TG, whereas consumption of glucose did not in healthy volunteers without obesity
and insulin resistance [32,34]. Therefore, the kind of food intake
significantly affects the formation of VLDL remnants and enhances the visceral
fat obesity in normal volunteers. Therefore, we have recognized that the role
for the formation of VLDL remnants after food intake is to provide TG as energy
supply to organs and tissues, in particular to adipose tissue to prepare
against starvation [34]. Further excessive and continuous supply of RLP from
the blood stream enhances the accumulation of TG in visceral fat. The enlarged
adipocytes by accumulated TG increase the secretion of TNF-α and other
adipocytokines [35] and induce insulin resistance. Takahashi
et al. [36] reported that VLDL receptor, which is actually the most important
VLDL remnant receptor, played the key role to induce the obesity and insulin
resistance when fed with high-fat refined-sugar (HFS) in mice. Although there
are many experimental animal studies that HFS can induce obesity and insulin resistance [37-42],
those literatures reported simultaneous
increase of TG or postprandial hyperlipidemia with insulin resistance
and obesity or TG increase after insulin resistance and obesity. Goudriaan et
al [43] reported that mice increased TG (VLDL remnants) in plasma by HFS, but
mice could not proceed to store TG in adipose tissue without VLDL receptor and
could not induce insulin resistance. Therefore, the formation of VLDL remnants
as the first step after food intake should be positioned before the obesity and
insulin resistance at the metabolic domino as the same position with blood
sugar (Figure 1). RLP
is known to be cleared by LRP-1 and VLDL receptors in the liver, muscle, endothelium
and adipose tissue in humans [36,41,42]. We recently found that LPL and apo (a)
(a part of Lp(a)) were bound to RLP and formed RLP-LPL and RLP-apo(a) complex
in plasma [1, 22]. Therefore, LPL and apo (a) as well as apoE could be the
ligands for RLP to bind remnant receptors, especially VLDL receptor in adipose
tissue. Elevated plasma RLP-C and RLP-TG concentration have been reported with
the presence of insulin resistance [44-46]. Also, Yatsuzuka et al. [47]
reported that RLP-C and RLP-TG were strongly associated with visceral fat
volume. This suggests the VLDL receptor in adipose tissue reacts with ligands
of RLP [37,41,42] and RLP supply FFA or incorporate into adipocytes. However,
the mechanism of incorporation of RLP into adipocytes is not yet clarified
enough. Thus, enlarged adipocytes by the increase of TG storage leads to the
induction of insulin resistance. Therefore, we suggest that after the intake of
high fat and refined sugar diet and/or with the lack of exercise, excessively
increased RLP in plasma cause to initiate obesity and insulin resistance
through VLDL receptor. Nordestgaard
et al. [48] and Bansal et al. [49] as well as Iso et al. [50] reported that the
TG measured in non-fasting samples were more sensitive than the conventional
measurements of the fasting TG concentrations in predicting the risk of cardiovascular events
(the Copenhagen Heart Study, Womens Health Study and in a Japanese population
study). Also, the Framingham Offspring Study previously cardiovascular risk
factor, while RLP-C was an independent risk factor in the fasting plasma in
women[51]. Postprandial
TG and RLP are known to attain their highest levels 3-6 h after food intake
[7-9]. Therefore, we analyzed the RLP-TG/TG ratio (concentration) and
RLP-TG/RLP-C ratio (particle size) [10] associated with the lipoprotein lipase
(LPL) in both the fasting and postprandial plasma. We have shown that
significantly higher RLP-TG is contained in the postprandial plasma than
fasting plasma when the TG level is adjusted as the RLP-TG/TG ratio [18]. These
results show that the amount and ratio of RLP in the postprandial TG increased
significantly compared with the fasting plasma TG. Karpe et al. [29,30]
reported that endogenous TG-rich lipoproteins (TRL) accumulate in the human
plasma after fat intake and the mechanism behind this phenomenon is the delayed
lipolysis of the apoB-100 TRL particles due to a competition with CM for the
LPL active sites. The large apoB100 TRL is more atherogenic than small TRL.
Postprandial RLP with large particle size and low LPL are atherogenic
lipoproteins and cause higher risk of cardiovascular diseases. We
have shown that postprandial VLDL remnants are increased significantly after
fat rich meal or consuming fructose. Elevated VLDL remnants are significantly
large sized RLP particles along with a smaller ratio of LPL in RLP in the
postprandial plasma. Therefore, we have proposed that the increase of
postprandial VLDL remnants comes first associated with unhealthy life style
habit and cause to induce the insulin resistance as the results of obesity when
the excessive RLP is supplied continuously to visceral adipocytes. Therefore,
metabolic domino starts from the increased postprandial VLDL remnants in plasma
as shown in Figure 1 and cause various cardiovascular diseases followed by the
obesity and insulin resistance. The Japanese cuisine is an ideal food which
increase low amount of VLDL remnants. 1.
Nakajima
K, Tokita Y and Tanaka A. Hypothesis: Postprandial remnant lipoproteins are the
causal factors that induce the insulin resistance associated with obesity
(2018) Clin Chim Acta 485: 162-132. https://doi.org/10.1016/j.cca.2018.06.029 2.
Woods
SC. Signals that influence food intake and body weight (2005) Physiol Behav 86:
709 –716. https://doi.org/10.1016/j.physbeh.2005.08.060 3.
Zilversmit
DB. Atherogenesis: a postprandial phenomenon (1979) Circulation 60: 473–485. 4.
Nagao
T, Watanabe H, Goto N, Onizawa K and Taguchi H. Dietary diacylglycerol
suppresses accumulation of body fat compared to triacylglycerol in men in a
double-blind controlled trial (2000) J Nutr. 130: 792-797. https://doi.org/10.1093/jn/130.4.792 5.
St-Onge
MT and Jones PJ. Physiological effects of medium-chain
triglycerides: potential agents in the prevention of obesity (2002) J Nutr.
132: 329-332. https://doi.org/10.1093/jn/132.3.329 6.
Cohn
JS, Marcoux C and Davignon J. Detection, quantification, and characterization
of potentially atherogenic triglyceride-rich remnant lipoproteins (1999) Arterioscler Thromb Vasc Biol 19: 2474-2486. 7.
Nakajima
K, Saito AT, Tamura M. Suzuki T, Nakano M, et al. Cholesterol in remnant-like
lipoproteins in human serum using monoclonal anti apo B-100 and antiapo A-I
immunoaffinity mixed gel (1993) Clin Chim Acta 223: 53-71. https://doi.org/10.1016/0009-8981(93)90062-9 8.
Nakajima
K, Okazaki M, Tanaka A, Pullinger CR, Wang T, et al. Separation and
determination of remnant-like particles in serum from diabetes patients using
monoclonal antibodies to apo B-100 and apo A-I (1996) J Clin Ligand Assay 19:
177-183. 9.
Nakano
T, Tokita Y, Nagamine T, Tanaka A, Okazaki M, et al. Measurement of serum
remnant-like lipoprotein particle-triglyceride (RLP-TG) and RLP-TG/ total TG
ratio using highly sensitive triglyceride assay reagent (2011) Clin Chim Acta 412: 71-78. https://doi.org/10.1016/j.cca.2010.09.040 10.
Nakano
T, Tanaka A, Okazaki M, Tokita Y, Nagamine T, et al. Particle size of apoB-48
carrying lipoproteins in remnant lipoproteins isolated from postprandial plasma
(2011) Ann Clin Biochem 48: 57–64.
https://doi.org/10.1258%2Facb.2010.010193 11.
Nakajima
K, Nakano T, Tokita Y, Nagamine T, Yatsuzuka S, et al. The characteristics of
remnant lipoproteins in the fasting and postprandial plasma (2012) Clin Chim Acta 413 1077-1086. https://doi.org/10.1016/j.cca.2012.02.026 12.
Nakajima
K and Tanaka A. Atherogenic postprandial RLP;VLDL remnants as
a causal factor in atherosclerosis (2018) Clin Chim Acta. 478: 200-215. https://doi.org/10.1016/j.cca.2017.12.039 13.
Nakajima
K and Tanaka A. Postprandial remnant lipoproteins as targets for the prevention
of atherosclerosis (2018) Curr Opin Endocrinol Diabetes Obes 25: 108-117 14.
Nakajima
K, Nagamine T, Fujita MQ, Tanaka A and Schaefer E. Apolipoprotein B-48: A
Unique Marker of Chylomicron Metabolism (2014) Advances in Clinical Chemistry,
Burlington:Academic Press 64:117-177. https://doi.org/10.1016/B978-0-12-800263-6.00003-3 15.
Schneeman
BO, Kotite L, Todd KM and Havel RJ. Relationships between the responses of
triglyceride-rich lipoproteins in blood plasma containing apolipoprotein B-48
and B-100 to a fat-containing meal in normolipidemic humans (1993) Proc Natl
Acad Sci 90: 2069-2073. 16.
Nestel
PJ. Relationship between plasma triglycerides and removal of chylomicrons
(1964) J Clin Invest 43: 943–949. https://doi.org/10.1172/JCI104980 17.
Grundy
SM and Mok HY, Chylomicron clearance in normal and hyperlipidemic man (1976)
Metabolism 25: 1225–1239. 18.
Nakajima
K, Tokita Y, Sakamaki K, Shimomura Y,
Kobayashi J et al. Triglyceride content in remnant lipoproteins is
significantly increased after food intake and is associated with plasma
lipoprotein lipase (2017) Clin Chim Acta
465: 45-52. https://doi.org/10.1016/j.cca.2016.12.011 19.
Marcoux
C, Hopkins PN, Wang T, Elizabeth TL, Nakajima K et al. Remnant-like particle
cholesterol and triglyceride levels of hypertriglyceridemic patients in the fed
and fasted state (2000) J Lipid Res 41: 1428-1436. 20.
Ooi
TC, Cousins M, Ooi DS, Steiner G, Nakajima K, et al. Postprandial remnant- like
lipoproteins in hypertriglyceridemia (2001) J Clin Endocrinol Metab 86: 3134-3142.
https://doi.org/10.1210/jcem.86.7.7627 21.
Nakajima
K, Nakano T, Moon HD, Nagamine T, Stanhope KL et al. The correlation between TG
vs remnant lipoproteins in the fasting and postprandial plasma of 23 volunteers
(2009) Clin Chim Acta 404: 124-137. https://doi.org/10.1016/j.cca.2009.03.051 22.
Sato
K, Okajima F, Miyashita K, Kobayashi J, Stanhope KL, et al. Majority
of lipoprotein lipase is bound to remnant lipoproteins in plasma: A new
definition for remnant lipoproteins (2016)
Clin Chim Acta 46: 114-125 https://doi.org/10.1016/j.cca.2016.06.020 23.
Ishiyama
N, Nakajima K, Sakamaki K, Tokita Y, Tanaka A et al. Lipoprotein lipase does
not increase in postprandial plasma (2017) Clin Chim Acta 464 204-210. https://doi.org/10.1016/j.cca.2016.11.035 24.
Beigneux
AP, Davies BS, Gin P, Weinstein MM, Farber E, et al.
Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein
1 plays a critical role in the lipolytic processing of chylomicrons (2007) Cell Metab 5: 279 –291. https://doi.org/10.1016/j.cmet.2007.02.002 25.
Khovidhunkit
W, Charoen S, Kiateprungvej A, Chartyingcharoen P, Muanpetch S, et al. Rare and
common variants in LPL and APOA5 in Thai subjects with severe
hypertriglyceridemia: A resequencing approach (2016) J Clin Lipidol 10: 505-511. https://doi.org/10.1016/j.jacl.2015.11.007 26.
Shirakawa,
T, Nakajima K, Shimomura Y, Kobayashi J, Stanhope K, et al. Comparison of the
effect of post-heparin and pre-heparin lipoprotein lipase and hepatic
triglyceride lipase on remnant lipoprotein metabolism. Clinica Chimica Acta 440
(2014) 193-200. https://doi.org/10.1016/j.cca.2014.07.020 27.
Shirakawa
T, Nakajima K, Yatsuzuka S, Shimomura Y, Kobayashi J, et al. The role of
circulating lipoprotein lipase and adiponectin on the particle size of remnant
lipoproteins in patients with diabetes mellitus and metabolic syndrome (2014)
Clinica Chimica Acta 440: 123-132. https://doi.org/10.1016/j.cca.2014.10.029 28.
Beisiegel
U, W Weber and G Bengtsson-Olivecrona. Lipoprotein lipase enhances the binding
of chylomicrons to low density lipoprotein receptor-related protein (1991) Proc
Natl Acad Sci 88: 8342-8346. https://doi.org/10.1073/pnas.88.19.8342 29.
Karpe
F. Postprandial lipoprotein metabolism and atherosclerosis (1999) J Internal Med 246: 341-355. https://doi.org/10.1046/j.1365-2796.1999.00548.x 30.
Karpe
F, Steiner G, Olivecrona T, Carison LA and Hamsten A. Metabolism of
triglyceride-rich lipoproteins during alimentary lipemia (1993) J Clin Invest
91: 748-758. 31.
Rip
J, Nierman MC, Wareham NJ, Luben R, Bingham SA, et al. Serum lipoprotein lipase
concentration and risk for future coronary artery disease: the EPIC-Norfolk
prospective population study (2006)
Arterioscler Thromb Vasc Biol 26: 637-642. https://doi.org/10.1161/01.ATV.0000201038.47949.56 32.
Stanhope
KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al . Consuming
fructose-sweetened, not glucose-sweetened, beverages increases visceral
adiposity and lipids and decreases insulin sensitivity in overweight/obese
humans. J Clin Invest. 119 (2009) 1322-1334 33.
Stanhope
KL, Bremer AA, Medici V, Nakajima K, Ito Y,et al. Consumption of fructose and
high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol,
and apolipoprotein-B in young men and women (2011) J Clin Endocrinol Metab 96: 596-605. https://doi.org/10.1210/jc.2011-1251 34.
Stanhope
KL, Medici V, Bremer AA, Lee V, Lam HD, et al. A dose-response study of
consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein
risk factors for cardiovascular disease in young adults (2015) Am J Clin Nutr
101: 1144-1154. https://doi.org/10.3945/ajcn.114.100461 35.
Hotamisligil
GS, Shargill NS and Spiegelman BM. Adipose expression of tumor necrosis
factor-alpha: direct role in obesity-linked insulin resistance (1993) Sci 259: 87-91. 36.
Takahashi
S. Triglyceride Rich Lipoprotein -LPL-VLDL Receptor and Lp(a)-VLDL Receptor
Pathways for Macrophage Foam Cell Formation (2017) J Atheroscler Thromb 24: 552-559. 37.
Oscai
LB, Miller WC and Arnall DA. Effects of dietary sugar and of dietary fat on
food intake and body fat content in rats (1987) Growth 51: 64–73. 38.
Oscai
LB, Brown MM and Miller WC. Effect of dietary fat on food intake, growth and
body composition in rats (1984) Growth 48: 415–424. 39.
Xiao-Bing
Cui, Jun-Na Luan, Jianping Ye and Shi-You Chen. RGC32 deficiency protects
against high-fat diet-induced obesity and insulin resistance in mice (2015) J
Endocrinol 224: 127–137. https://doi.org/10.1530/JOE-14-0548 40.
Barnard
RJ, DJ Faria, JE Menges, and DA Martin. Effects of a high-fat, sucrose diet on
serum insulin and related atherosclerotic risk factors in rats (1993) Atherosclerosis 100: 229 – 236. https://doi.org/10.1016/0021-9150(93)90209-D 41.
Barnard
RJ, DA Martin, EJ Ugianskis and SB Inkeles. Role of diet and exercise in the
management of hyperinsulinemia and associated atherosclerotic risk factors
(1992) Am J Cardiol 69: 440–444. https://doi.org/10.1016/0002-9149(92)90981-4 42.
Takahashi S, Sakai J, Fujino T, Hattori
H, Zenimaru Y, et al. The very low-density lipoprotein (VLDL) receptor:
characterization and functions as a peripheral lipoprotein receptor (2004) J
Atheroscler Thromb 11: 200-208. 43.
Goudriaan RJI, Tacken PJ, Dahlmans VE,
Gijbels MJ, van Dijk KW, et al. Protection from obesity in mice lacking the
VLDL receptor (2001) Arterioscler Thromb
Vasc Biol 21: 1488-1493. 44.
Imagawa
M, Takahashi S, Zenimaru Y, Kimura T, Suzuki J, et al. Comparative reactivity
of remnant-like lipoprotein particles (RLP) and low-density lipoprotein (LDL)
to LDL receptor and VLDL receptor: effect of a high-dose statin on VLDL
receptor expression (2012) Clinica Chimica acta 413: 441- 447. https://doi.org/10.1016/j.cca.2011.10.033 45.
Chan
DC, Watts GF, Barrett PH, Mamo JC and Redgrave TG. Markers of triglyceride-rich
lipoprotein remnant metabolism in visceral obesity (2002) Clinica Chemica Acta 48: 278-283. 46.
Ohnishi
H, Saitoh S, Takagi S, Ohata J, Isobe T, et al. Relationship between
insulin-resistance and remnant-like particle cholesterol (2002) Atherosclerosis
164: 167-170. https://doi.org/10.1016/S0021-9150(02)00057-6 47.
Yatsuzuka
S, Shimomura Y, Akuzawa M, Ando Y, Kobayashi J, et al. Plasma adiponectin is a
more specific marker of fatty liver than a marker of metabolic syndrome in
Japanese men (2014) Ann Clin Biochem 51: 68–79. https://doi.org/10.1177%2F0004563213487892 48.
Nordestrgaad
BG, Benn M, Schnohr P and Tybjaerg-Hansen A. Nonfasting triglycerides and risk
of myocardial infarction, ischemic heart disease, and death in men and women
(2007) JAMA 298: 299-308. 49.
Bansal
S, Buring JE, Rifai N, Mora S, Sacks FM, et al. Fasting compared with
nonfasting triglycerides and risk of cardiovascular events in women (2007) JAMA 298: 309-316. 50.
Iso
H, Naito Y, Sato S, Kitamura A, Okamura T, et al. Serum triglycerides and risk
of coronary heart disease among Japanese men and women (2001) Am J Epidemiol
153: 490-499. https://doi.org/10.1093/aje/153.5.490
McNamara JR, Shah PK,
Nakajima K, Cupples LA, Wilson PW, et al. Remnant-like particle (RLP)
cholesterol is an independent cardiovascular disease risk factor in women:
results from the Framingham Heart Study (2001)
Atherosclerosis 154: 229- 236. *Corresponding author:Katsuyuki Nakajima, Graduate
School of Health Sciences, Gunma University, 3-47-4, Minami-Cho, Maebashi,
Gunma, Japan(371-0805),
Tel: +81-27-223-7267, Fax No: +81-27-223-7267, E-mail: nakajimak05@ybb.ne.jp
Citation: Nakajima
K, Tokita Y and Tanaka A. Triglyceride is significantly increased in remnant
lipoproteins after food intake and its association with lipoprotein lipase in
the plasma (2018) J Obesity and Diabetes 2: 6-10Triglyceride is Significantly Increased in Remnant Lipoproteins After Food Intake and its Association with Lipoprotein Lipase in the Plasma
Abstract
Full-Text
Why TG increase
after food intake?
Which TG in
lipoproteins increase after food intake?
What percentage
of TG is comprised of postprandial RLP-TG?
How the increased
TG is metabolized by LPL?
The increase of
postprandial RLP is the result of obesity and insulin resistance or cause of
them?
Why postprandial
TG is a risk of cardiovascular diseases?
Conclusion
References
Keywords