Research Article :
Type
2 Diabetes Mellitus (T2DM) is a major health concern globally. The total
number of Diabetics is expected to reach 366 million by 2030 [1]. The
prevalence of T2DM in Saudi Arabia is one of the highest reported in the world,
reaching up to 30% [2]. Vitamin D deficiency remains a major health problem in
many parts of the world [3]. The main marker of vitamin D status is the metabolite
25-hydroxyvitamin D (25(OH)D) [4,5] It is now increasingly recognized that
vitamin D deficiency is defined as serum 25(OH)D concentration <50 nmol/L [5].
The prevalence of vitamin D deficiency in the general world population
including Saudi Arabia is as high as 50-80% [6-9].Evidence
suggests a link between vitamin D deficiency and T2DM [10-15]. The prevalence
of vitamin D deficiency in patients with T2DM
varies from 70 to 90%, depending on the threshold used to define vitamin D
deficiency [16,17]. It has been postulated that vitamin D has an influence on
glycemic control [18]. Pancreatic
beta cell function may be affected by the existence of specific vitamin D
receptors in the beta cells [19]. Additionally, vitamin D is essential for
pancreatic β cells insulin
secretion regulation and calcium absorption [20]. It is thought that
vitamin D stimulates glucose transport and preventing systemic inflammation
[21,22]. Few published researches have surveyed the prevalence of vitamin D deficiency
in Saudi patients with T2DM and the correlation between Vitamin D status and glycaemic
control [23]. Hence the present study was conducted to investigate the
status of vitamin D and its correlation with glycated haemoglobin in type
2 diabetes mellitus. A
cross-sectional single centre study was conducted in 2440 patients with T2DM attending
the Diabetes Centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia
between January 2018 and December 2018. Eligible patients were 20 years or
older. Exclusion criteria were known hepatic or renal disease, metabolic bone
disease, malabsorption, hypercortisolism,
malignancy, immobility for more than one-week, pregnancy, lactation, and
medications influencing bone metabolism.
The serum concentration of 25(OH)D was measured by competitive protein
binding assay using kits (Immunodiagnostic, Bensheim, Germany). Vitamin D
deficiency was defined as serum 25-OHD concentration <50 nmol/L.3 Glycosylated
hemoglobin (HbA1c) was measured by the high performance liquid
chromatography method (Bio-Rad Laboratories, Waters, MA, USA). The total numbers
of cohort were separated on basis of age values into five groups: 20-29 years,
30-40 years, 40-49 years, 50-59 years and ≥60 years. The study was approved by
the ethical committee board of King Fahad Armed Forces Hospital. Data
are presented as means ± Standard Deviation (SD) or numbers (%). Quantitative
variables were compared between two groups by using the Students test.
Differences in categorical variables were analysed using the chi-square test.
The relationship between continuous variables was assessed using coefficients
of correlation. P value <0.05 indicates significance. The statistical
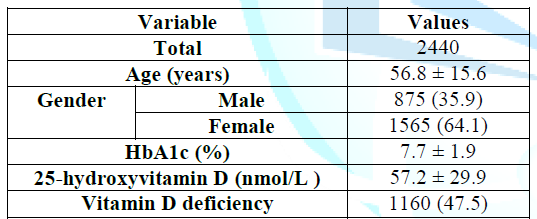
analysis was conducted with SPSS version 23.0 for Windows. Table 1: Patient
characteristics (mean±standard deviation or number (%)) The
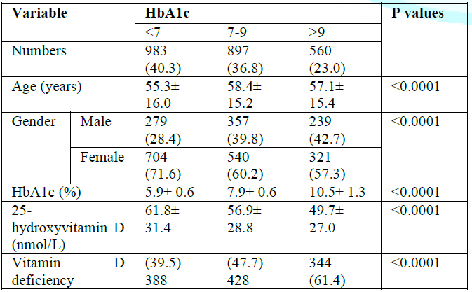
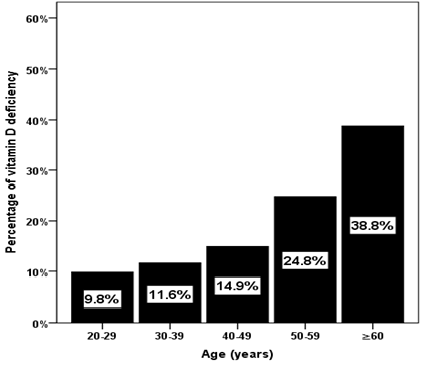
frequency of vitamin D deficiency was upward as age advanced (Figure 1). The frequency of vitamin D
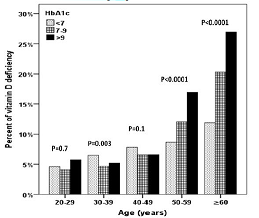
deficiency was upward across HbA1c groups as age advanced with highest
frequency of vitamin D deficiency was found to be statistically significant in
HbA1c>9% compared to HbA1c< and 7%-9% groups in the age group 50-59 years
and ≥60 years (Figure 2) with males
most frequently predominant than females in all age group associated with HbA1c
7%-9% and 9% (Figure 3). HbA1c was
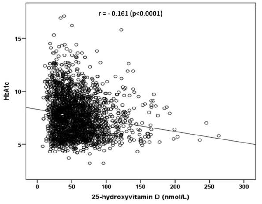
significantly positively correlated with age (r= 0.055, p=0.007) (Figure 4) whereas 25-OHD concentration was significantly negatively
correlated with age (r=-0.161, p<0.0001) (Figure 5). Figure 1: The percentage
of vitamin D deficiency in relation to
age groups Figure 2: The percentage
of vitamin D deficiency in relation to HbA1c in correlation to age groups Figure 4: Scatter plot
indicating negative correlation between HBA1C levels witt age Figure 5: Scatter plot
indicating negative correlation between HBA1C levels with Vitamin D levels Diabetes
mellitus is currently the most prevalent chronic illness in the world having a
prevalence of around 9% in the adult population amd 30% in Saudi Arabia [2,24].
Vitamin D deficiency plays an important role in development of T2DM [25] We
detected a significant negative correlation between plasma
vitamin D and each of HbA1c levels in all studied groups in harmony with
others [26-29]. It is of importance to state that the sample size is
representative for a number of subjects suffering from T2DM in the area and
study population of one institution does not represent the entire city of
Jeddah, in addition the study sample confined to patients with T2DM but without
comparable groups. In
our study around 48% of the subjects were vitamin D deficient with a mean
25-OHD level of 57 nmol/l. Bashir et al reported that 81% of the studied
subjects were vitamin D deficient with a mean 25-OHD level of 39nmol/l [30].
The causes of vitamin D deficiency could be due to changing life style with
people adopting a more sedentary life, little exposure to sunlight, reduced
outdoor activity, changes in dietary habits, carbohydrate and saturated
fat enhanced diet. These factors also contribute to both development of
T2DM and poor control of diabetes. Vitamin D deficiency has received special attention lately because of its high
incidence and its implication in the genesis of multiple
chronic illnesses. The high prevalence of vitamin D deficiency in our study
population underlines the fact that vitamin D deficiency is more common in
chronic diseases like diabetes mellitus. Our study showed that vitamin D was
inadequate in a half of our population of patients with T2DM. Lower vitamin D
levels were associated with a poor
glycemic control. This was more strongly associated with HbA1c
(p<0.0001). The study indicates a poor glycemic control (>9%) in a
majority (61%) of patients compared to 40% patients with good glycemic
control (7%). In patients having HbA1c greater than 7.0 vitamin D
deficiency was significantly greater (67%) compared to 33% patients with good
glycemic control (HbA1c<7) p<0.0001.There was a stronger co-relation
between HbA1c levels and serum 25-OHD levels. These findings are supported by a
number of international studies. Some studies showed no association of a low
25-OHD levels with HbA1c levels.30 But inverse correlation between the level of
25-OHD and HbA1c is well known [31,32]. In many studies 25-OHD levels were low
in subjects having higher HbA1c values both in patients with diabetes
mellitus indicating that they are inversely related [14,16,34-36]. In our study, the prevalence of vitamin D deficiency was much higher among the
older age-group (39%), whereas serum
25(OH)D was statistically significant positively correlated with age r=0.193
(p<0.0001), in consistent with most studies whereas other studies reported
the higher prevalence of vitamin D deficiency among the young people [37-42].
The positive correlation of 25(OH)D to age is in disagreement with a study
carried out in the US, where severe vitamin
D deficiency was found to be more common among the young, and less common
among the elderly [43]. Growing scientific evidence has implicated vitamin
D deficiency in a multitude of chronic conditions including T2DM [41]. With
the growing prevalence of vitamin D deficiency across Saudi Arabia and its
association with these leading causes of mortality, it has become more
important than ever to delineate vitamin Ds role in the pathogenesis of these
diseases and use data to pinpoint established risk factors for vitamin D
deficiency. The relationship between vitamin D deficiency and diabetes
has long been explored, with growing evidence suggesting vitamin D deficiency
is a contributing factor to the development of T2DM [40]. We had several
limitations the study was done at one centre and was done at one point of time.
The study sample confined to patients with T2DM but without comparable groups.
We conclude that vitamin D deficiency and its inverse association with Glycated
Haemoglobin in type 2 Diabetes Mellitus have been established in many
studies. Such a finding was demonstrated in the present study. An interesting
avenue in this aspect would be to see if supplementing with vitamin
D can help improve glycemic control in diabetic
population. There
is no any financial support or relationships that may pose conflict of
interest. 1.
Wild
S, Roglic G, Green A, Sicree R and King H. Global Prevalence of Diabetes
Estimates for the year 2000 and projections for 2030 (2004) Diabetes Care 27:
1047-1053. https://doi.org/10.2337/diacare.27.5.1047 2.
Alqurashi
KA, Aljabri KS, Bokhari SA. Prevalence
of diabetes mellitus in a Saudi community (2011) Ann Saudi Med 31: 19-23.
https://doi.org/10.4103/0256-4947.75773 3.
Holick
MF. High prevalence of vitamin D inadequacy and implications for health (2006)
Mayo Clin Proc 81: 353-373. https://doi.org/10.4065/81.3.353 4.
Mathieu
C, Badenhoop K. Vitamin D and Type 1 diabetes mellitus: state of the art (2005)
Trends in Endo and Met. 16: 261-267. https://doi.org/10.1016/j.tem.2005.06.004 5.
Holick
MF. Vitamin D deficiency (2007) N Eng J Med 357: 266-281. https://doi.org/10.1056/NEJMra070553 6.
Gind
AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D
insufficiency in the US population, 1988-2004 (2009) Arch Intern Med 169: 626-632.
https://doi.org/10.1001/archinternmed.2008.604 7.
Maalouf
G, Gannage-Yared MH, Ezzedine J, Larijani B, Badawi S, Rached A, et. al. Middle
East and North Africa consensus on osteoporosis (2007) J Muskuloskelet Neuronal Interact 7: 131-143. 8.
Sedrani
SH, Elidrissy AW and El Arabi KM. Sunlight and vitamin D status in normal Saudi
subjects (1983) Am J Clin Nutr 38: 129-132.
https://doi.org/10.1093/ajcn/38.1.129 9.
Al-Turki
HA, Sadat-Ali M, Al-Elq AH, Al-Mulhim FA and Al-Ali AK. 25-Hydroxyvitamin D
levels among healthy Saudi Arabian women (2008) Saudi Med J. 29: 1765-1768. 10.
Ozfirat
Z and Chowdhury TA. Vitamin D deficiency and type 2 diabetes (2010) Postgrad
Med J 86: 18-25. https://doi.org/10.1136/pgmj.2009.078626 11.
Matilla
C, Knekt P, Mannisto, Rissanen H, Laaksonen MA, S et al. Serum
25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes (2007)
Diabetes Care 30: 2569-2570. https://doi.org/10.2337/dc07-0292 12.
Pittas
AG, Dawson- Hughes B, Li T et al. Vitamin D and calcium intake in relation to
type 2 diabetes in women (2006) Diabetes Care 29: 650-656. https://doi.org/10.2337/diacare.29.03.06.dc05-1961 13.
Thorand
B, Zierer A, Huth C, Linseisen J, Meisinger C, et al. Effect of serum
25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by
subclinical inflammation: results from the MONICA/KORA Augsburg study (2011) Diabetes
Care 34: 2320-2322. https://doi.org/10.2337/dc11-0775 14.
Cigolini
M, Iagulli MP, Miconi V, Galiotto M, Lombardi S,et al. Serum 25-hydroxyvitamin
D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic
patients (2006) Diabetes Care 29: 722-724. https://doi.org/10.2337/diacare.29.03.06.dc05-2148 15.
Scragg
R, Holdaway I, Singh V, Metcalf P, Baker J, et al. Serum 25-hydroxyvitamin D3
levels decreased in impaired glucose tolerance and diabetes mellitus (1995)
Diabetes Res Clin Pract. 27: 181-188. https://doi.org/10.1016/0168-8227(95)01040-K 16.
Tahrani
AA, Ball A, Shepherd L, Rahim A, Jones AF, et al. The prevalence of vitamin D
abnormalities in South Asians with type 2 diabetes mellitus in the UK (2010)
Int J Clin Pract 64: 351-355. https://doi.org/10.1111/j.1742-1241.2009.02221.x 17.
Miñambres
I, Sánchez-Quesada JL, Vinagre I, Sánchez- Hernández J, Urgell E, et al.
Hypovitaminosis D in type 2 diabetes: relation with features of the metabolic
syndrome and glycemic control (2014) Endocr Res 40: 160-165. https://doi.org/10.3109/07435800.2014.982326 18.
Parker
J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, et al. Levels of vitamin D and
cardiometabolic disorders; systematic review and meta-analysis (2009)
Maturitas. 65: 225-236. https://doi.org/10.1016/j.maturitas.2009.12.013 19.
Satin
LS, Butler PC, Ha J and Sherman AS. Pulsatile insulin secretion, impaired glucose
tolerance and type 2 diabetes (2015) Mol Aspects Med 42: 61-77. https://doi.org/10.1016/j.mam.2015.01.003 20.
Pittas
AG, Lau J, Hu FB and Dawson-Hughes B. Review: the role of vitamin D and
cal¬cium in type 2 diabetes. A systematic review and meta-analysis (2007) J Clin
Endocrinol Metab 92: 2017-2029. https://doi.org/10.1210/jc.2007-0298 21.
Johnson
JA, Grande JP, Roche PC and Kumar R. Immunohistochemical localization of the
1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas (1994) Am J Physiol 267:356‒360. https://doi.org/10.1152/ajpendo.1994.267.3.E356 22.
Kitaguchi
T, Oya M, Wada Y, Tsuboi T and Miyawaki A. Extracellular calcium influx
activa¬tes adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells
(2013) Biochem J 450: 365-373. https://doi.org/10.1042/BJ20121022 23.
Al-Zaharani
M and Majmaah. The prevalence of Vitamin D deficiency in Type 2 Diabetic
patients (2013) J health sciences 1: 18-22. 24.
World
Health Organization [Internet]. Global status report on non-communicable
diseases 2014. http://www.who.int/nmh/publications/ncd-status-report-2014/en 25.
Matthews
DR, Hosker JP, Rudenski AS and Naylor BA, Treacher DF. Homeostasis model assessment:
insulin resistance and beta cell function from plasma FBS and insulin
concentrations in man (1985) Diabetologia 28: 412-419. 26.
Schuch
NJ, Garcia VC, Vívolo SR and Martini LA. Relationship between vitamin D
receptor gene polymorphisms and the components of metabolic syndrome (2013)
Nutr J 12: 96. https://doi.org/10.1186/1475-2891-12-96 27.
Mukhopadhyaya
PN, Acharya A, Chavan Y, Purohit SS and Mutha A. Metagenomic study of single-nucleotide
polymorphisms within candidate genes associated with type 2 diabetes in an
Indian population (2010) Genet Mol Res. 9: 2060-2068. https://doi.org/10.4238/vol9-4gmr883 28.
Jiffri
EH and Al-Dahr MH. Vitamin d status and glucose hemostasis among Saudi type 2
diabetic patients (2017) J Diabetes Metab Disord Control 4: 110-114. https://doi.org/10.15406/jdmdc.2017.04.00117 29.
Bid
HK, Konwar R, Aggarwal CG, Gautam S and Saxena M. Vitamin D receptor (FolkI,
BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian
study (2009) Indian J Med Sci 63: 187-194. https://doi.org/10.4103/0019-5359.53164 30.
Bashir
F, Khan ZU, Qureshi S, Seetlani NK and Sheikh Z. Prevalence of Hypovitaminosis
D in Type 2 Diabetes Mellitus and its Relationship with Glycemic Control (2016)
J Liaquat Uni Med Health Sci 15: 83-89. 31.
Husemoen
LLN, Thuesen BH, Fenger M, Jorgensen T, Glumer C, et al. Serum 25 (OH)D and
Type 2 Diabetes Association in a General Population: A prospective study (2012)
Diabetes Care. 35: 1695-1700. https://doi.org/10.2337/dc11-1309 32.
Palomer
X, Gonzalez-Clemente J, Blanco-Vaca F and Mauricio D. Role of vitamin D in the
pathogenesis of type 2 diabetes mellitus (2008). Diabetes Obes Metab 10: 185-197.
https://doi.org/10.1111/j.1463-1326.2007.00710.x 33.
Boucher
BJ, Mannan N, Noonan K, Hales CN and Evans SJ. Glucose intoleranceand
impairment of insulin secretion in relation to vitamin D deficiency in east
LondonAsians (1995) Diabetologia 38: 1239-1245. 34.
Hutchinson
MS, Figenshau Y, NjølstadI, Schirmer H and Jorde R. Serum25-hydroxyvitamin D
levels are inversely associated with glycatedhaemoglobin (HbA(1c)).The
TromsøStudy (2011) Scand J Clin Lab Invest 71: 399-406. https://doi.org/10.3109/00365513.2011.575235 35.
Kositsawat
J, Freeman VL, Gerber BS and Geraci S. Association of A1Clevels with vitamin D
status in U.S. adults: data from the National Health andNutrition Examination
Survey (2010) Diabetes Care 33: 1236-1238. https://doi.org/10.2337/dc09-2150 36.
Nakhl
S, Sleilaty G, El Samad S, Saliba Y, Chahine R, et al. Association between
vitamin D deficiency and lipid and non-lipid markers of cardiovascular diseases
in the middle east region (2018) Eur J Clin Nutr. https://doi.org/10.1038/s41430-018-0280-1 37.
Burnard
B, Sloutskis D, Gianoli F, Cornuz J, Rickenbach M, et al. Serum
25-hydroxyvitamin D: distribution and determinants in the Swiss population
(1992) Am J Clin Nutr 56: 537-542. https://doi.org/10.1093/ajcn/56.3.537 38.
Lips
P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly:
consequences for bone wloss and fractures and therapeutic implications (2001)
Endocr Rev 22: 477-4501. https://doi.org/10.1210/edrv.22.4.0437 39.
Silva
Hovsepian, Massoud Amini, Ashraf Aminorroaya, Peyvand Amini and Bijan Iraj.
Prevalence of Vitamin D Deficiency among Adult Population of Isfahan City, Iran
(2011) J health popul nutr 29:149-155 40.
Martin
T. Campbell RK: Vitamin D and diabetes (2011) Diabetes Spectr 24: 113-118. 41.
Holick
MF. The vitamin D epidemic and its health consequences (2005) J Nutr 135: 2739-2748.
https://doi.org/10.1093/jn/135.11.2739S 42. Sadat-Ali M, Al-Elq
AM, Al-Turki H, Al-Mulhim
F and Al-Ali A. Vitamin D levels among Healthy Saudi Arabian Men (2009) Annals
of Saudi Med 29: 378-382. https://dx.doi.org/10.4103%2F0256-4947.55168 43.
Plotnikoff
GA and Quigley JM. Prevalence of severe hypovitaminosis D in patients with
persistent, nonspecific musculoskeletal pain (2003) Mayo Clin Proc 78:
1463-1470. https://doi.org/10.4065/78.12.1463 Corresponding author: Khalid
S Aljabri, Department
of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi
Arabia, Tel: +966590008035, E-mail: khalidsaljabri@yahoo.com
Citation: Aljabri KS, Alnasser IM, Bokhari SA, Alshareef MA, Khan PM, et al. Study
of vitamin D status and its correlation with glycated haemoglobinin type 2
diabetes mellitus (2019) J Obesity and Diabetes 3: 12-16 Type 2 Diabetes mellitus, Glycated Haemoglobin
and Vitamin D statusStudy of Vitamin D Status and its Correlation with Glycated Haemoglobinin Type 2 Diabetes Mellitus
Khalid S Aljabri
Abstract
Introduction: Few published researches have surveyed the
correlation between Vitamin D status and glycaemic control in type 2 diabetes
mellitus (T2DM). The present study was conducted to investigate the status of
vitamin D and its correlation with glycated
haemoglobin in type 2 diabetes mellitus.
Method: A cross-sectional single
centre study was conducted in 2440 patients with T2DM attending the Diabetes
Centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia between
January 2018 and December 2018. Eligible patients were 20 years or older.
Results: There were 2440 patients
with T2DM. Vitamin D deficiency (25-OHD<50 nmol/l) was found 47.5%. Patients
with HbA1c<7% were younger than patients with HbA1c 7%-9% and >9% (55.3
±16.0 vs. 58.4 ±15.2 vs. 57.1 ±15.4 respectively, p<0.0001). The mean 25-OHD
concentration was statistically significant lower in patients with Hba1c>9% compared
to patients with Hba1c<7% and 7%-9% (49.7 ±27.0 vs. 61.8 ±31.4 vs. 56.9
±28.8 respectively, p<0.0001). The frequency of vitamin D deficiency was
statistically significant higher in patients with Hba1c>9% compared to
patients with Hba1c<7% and 7%-9% (40% vs. 48% vs. 61% respectively,
p<0.0001). The frequency of vitamin D deficiency was upward across HbA1c
groups as age advanced with highest frequency of vitamin D deficiency was found
to be statistically significant in HbA1c>9% compared to HbA1c< and 7%-9%
groups in the age group 50-59 years and ≥60 years with males most frequently
predominant than females in all age group associated with HbA1c 7%-9% and 9%.
HbA1c was significantly positively correlated with age whereas 25-OHD
concentration was significantly negatively correlated with age.
Conclusions: We report vitamin D
deficiency and its inverse association with Glycated Haemoglobin in type 2
Diabetes Mellitus.
Full-Text
Introduction
Methods


Discussion
Acknowledgments
Keywords