Research Article :
Kaempferol is a polyphenolic compound and are widely
distributed in plants. It is used in the treatment of different disease
conditions. With the endemic resistant parasites against most antitrypanosomal
agents and the toxicity associated with diminazene aceturate, the search for
safer and more effective alternative therapy of trypanosomosis becomes
paramount. In this study the effect of treatment with kaempferol and diminazene
aceturate on Hematological parameters in mice with experimental Trypanosoma brucei brucei infection was
evaluated. Thirty six adult
swiss albino mice of either sex were randomly divided into six groups of six
mice each.Mice
in group I were untreated uninfected. Mice in group II were pre-treated with
kaempferol (1mg/kg) for 14 days. Mice
in groups II to VI each were inoculated with blood containing Trypanosoma brucei brucei (106
trypanosomes/ml of blood/animal) intraperitoneally. Following establishment of
the infection (four days post-inoculation), mice in group III were treated once
with diminazene aceturate (3.5 mg/kg) I.P. Mice in group IV were treated with
diminazene aceturate (3.5 mg/kg) once I.P, and then continued with kaempferol
(1 mg/kg) for nine days. In
Africa, trypanosomosis
is one of the neglected parasitic diseases of animal and human, which accounts
for the low livestock productivity (Welburn et al., 2006). About 70 million
people distributed over 1.55million km2 (Simarro et al., 2012), on
the other hand, about 250 million animals distributed over approximately 25
million km2 in Africa are at risk of the disease (WHO, 2006). The
pathogenesis of African trypanosomosis is partly due to the generation of
reactive oxygen species (ROS) due to stress induced by the parasite, which
causes degenerative changes in cells, tissues and organs of the infected
animals (Kobo et al., 2014).The ROS attack both the membrane polyunsaturated
fatty acids and proteins of RBCs, leading to oxidative hemolysis and
consequently, anemia as well as the depletion of endogenous antioxidant
reserved in the blood and other tissues of trypanosome-infected animals (Kobo
et al., 2014). The interplay of several factors acting either individually or
synergistically also contributes to the development of hemolytic anemia in
human and animal trypanosomosis, most common among these factors are
erythrocyte injury caused by lashing action of trypanosome flagella, platelet
aggregation, toxins and metabolites produced by trypanosomes, lipid
peroxidation and malnutrition (Murray and Morrison, 1978; Morrison et al., 1981;
Saror, 1982; Igbokwe, 1994). Chemotherapy and chemoprophylaxis
by trypanocides using isometamidium, homidium and diminazene, formed the most
important aspect of control and eradication of trypanosomosis in animals (Leach
and Roberts,1981; Kinabo, 1993; Anene, et. al. 2001). Reports from East, South
and West Africa (Ndungu et al., 1999; McDermott et al., 2000; Maikai et al.,
2007) have shown that the prevalence of
trypanocidal drug resistance to be very high. Antioxidants are
substances that protect cells from the damage caused by unstable molecules known
as free radicals (Kobo et al., 2014). Antioxidants scavenge free radicals and
prevent tissues and organs from damage caused by free radicals (Shimelis et
al., 2015). In the present study, the effects of treatment with kaempferol
and/or diminazene aceturate on hematological parameters of mice with
experimental Trypanosoma
brucei brucei infection was investigated using established procedures. Location of the
Research The
research was conducted in the Departments of Veterinary Pharmacology and
Toxicology and Parasitology
and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University
(A.B.U), Zaria, Kaduna State, Nigeria. Experimental
Animals Thirty
six adult Swiss albino mice of either sex weighing between 18 and 22 grams were
used in this study. The mice were reared in the animal house, Department of
Pharmacology and Toxicology, Faculty of Veterinary Medicine, Ahmadu Bello
University Zaria. These animals were housed in locally fabricated mice cages at
room temperature, 25°C. Wood shavings were used as beddings and changed once
every week. The experimental mice were allowed free access to rat chow and
water ad-libitum. All
animal experiments were carried out according to international guidelines as
approved by the postgraduate (ethical) committee of the Faculty of Veterinary
Medicine, Ahmadu Bello University Zaria. The Parasite Trypanosoma
brucei brucei
was obtained from the National Veterinary Research Institute (N.V.R.I) Vom,
Jos, Plateau State, Nigeria. The parasite was maintained by continuous passage
in a donor mouse. Parasitaemia was monitored by use of wet mount viewed under ×
400 magnifications (Herbert and Lumsden, 1976). Drug,
Sources and Preparation Kaempferol
was sourced from whitehead scientific (pty) limited, South Africa, it came
along with the following details; CAS number (520-18-3), Catalog number (3603),
EC number (208-287-6) and batch number (3). Diminazene aceturate was purchased
from pharmacy unit of the Veterinary Teaching Hospital (VTH), Faculty of
Veterinary Medicine, Ahmadu Bello University (A.B.U), Zaria, Kaduna State,
Nigeria. The drugs
(kaempferol and diminazene aceturate) were dissolved in distilled water and
administered to each mouse according to the body weight. The concentrations of
kaempferol and diminazene aceturate used were 0.5 mg/ml and 3 mg/12.5 ml,
respectively. Experimental
Infection of the Mice Trypanosomes
infected blood was obtained from the tail of the infected donor mice at peak of
Parasitaemia (109) and used to maintain parasite suspension in physiological
saline. The mice were inoculated (1 mL/mice) intraperitoneally with a
suspension, containing 3 or 4 trypanosomes per view at × 100 magnification
(approximately 106 trypanosomes per mL) as described by Ekanem and Yusuf
(2008). The Thirty six adult mice were
randomly divided into six groups of six mice each and were treated as follows: Group I- Mice in group
I were neither infected nor treated. Group II- Mice in group
II were pre-treated individually with kaempferol (1 mg/kg per os) for 14 days
and then infected with Trypanosoma brucei
brucei (106 trypanosomes/ml of blood). Group III- Mice in group
III were infected with Trypanosoma brucei
brucei (106 trypanosomes/ml of blood), after detection of Parasitaemia,
each mouse was treated once with diminazene aceturate (3.5 mg/kg i.p). Group IV- Mice in group
IV were infected with Trypanosoma brucei
brucei (106 trypanosomes/ml of blood), after detection of Parasitaemia,
each mouse was treated once with diminazene aceturate (3.5 mg/kg)
intraperitoneally, and then continued with kaempferol
(1 mg/kg per os) for 9 consecutive days. Group V- Mice in group
V were infected with Trypanosoma brucei
brucei (106 trypanosomes/ml of blood i.p), after detection of Parasitaemia,
they were treated with kaempferol (1 mg/kg per os) for 9 consecutive days. Group VI- Mice in group
VI were infected with Trypanosoma brucei
brucei (106 trypanosomes/ml of blood i.p) and then administered normal
saline at (5 ml/kg per os) for 9 consecutive days. The
Parasitaemia in the infected and treated groups was monitored daily using the
rapid matching counting method (Herbert and Lumsden, 1976). Hematological
Analysis At
the end of the experiment, mice from each group were sacrificed by severing the
jugular vein. About 1.5 ml of blood was collected from each mouse in a 20 ml
vial containing EDTA as an anti-coagulant and used to determine the Hematological
parameters; packed cell volume (PCV), total erythrocyte count, hemoglobin
concentration, and total and differential leucocyte counts were determined using methods
described by Dacie and Lewis (1991). The method descried by Schalm et al (1975)
was also used to calculate erythrocytic indices from PCV, hemoglobin
concentration and erythrocyte count. Statistical
Analysis Data
obtained was expressed as mean ± standard error of mean (S.E.M) and then
subjected to one-way analysis of variance (ANOVA) and compared with Turkey post-hoc test, using Graph Pad
Prism version 5.0 for windows (Graph Pad Software, San Diego, California, USA).
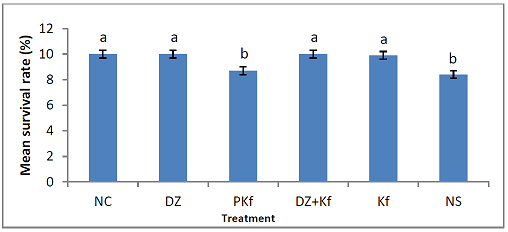
The level of significance was set at p< 0.05. Mean survival
rate Figure 1 shows the
effect of treatments with kaempferol and/ or diminazene
aceturate on mean survival rate in mice with experimental Trypanosoma brucei brucei infection. Mean survival rate
significantly (P < 0.001) decreased in mice pre-treated with kaempferol (group
II) and those that were infected and administered normal saline (group VI) when
compared to mice infected and treated with diminazene aceturate (group III),
diminazene aceturate and kaempferol (group IV) and kaempferol only (group V). Effects of
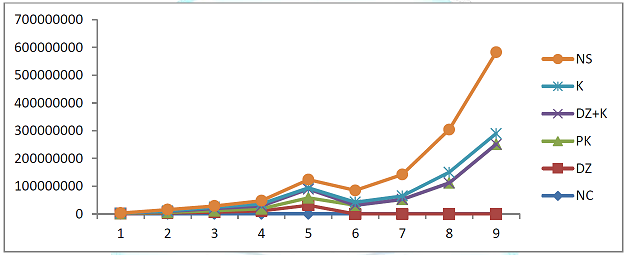
Treatments on the Level of Parasitaemia in Infected Mice The
effects of treatments with kaempferol and/ or diminazene aceturate in mice
experimentally infected with Trypanosoma
brucei brucei on the level of Parasitaemia is shown in Figure 2. Parasitaemia
increased progressively in mice pre-treated with kaempferol (group II), treated
with kaempferol only (group V) and those that were administered normal saline
(group VI) up to day 9 post infection. The parasites were cleared completely
from the blood stream of mice treated with diminazene aceturate only (group
III) and those that were treated with diminazene aceturate and kaempferol
(group IV) on day six post infection, hence remain aparasitaemic. Although the
onset of Parasitaemia was not different from other groups, but there was
significant (P< 0.001) reduction in the level of Parasitaemia in mice
treated kaempferol (group V) when compared to mice pre-treated with kaempferol
(group II) and those that were infected and administered normal saline (group
VI). Effects of Treatments on Hematological
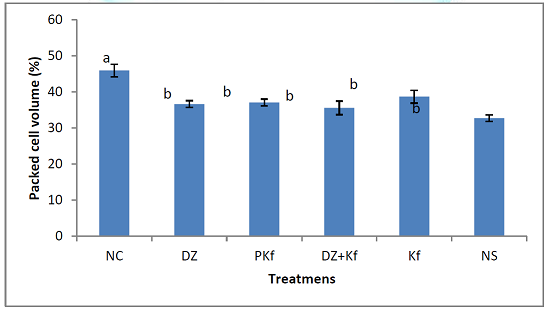
Parameters Packed Cell
Volume: Figure 3
shows the effects of treatments with kaempferol and/ or diminazene aceturate on
packed cell volume in mice infected with T.
brucei brucei. The mean packed cell volume increased significantly (P <
0.001) in mice treated with diminazene aceturate only (groups III), kaempferol
and diminazene
aceturate (group IV), kaempferol only (group V) when compared to mice
pre-treated with kaempferol (group II) and those that were infected and
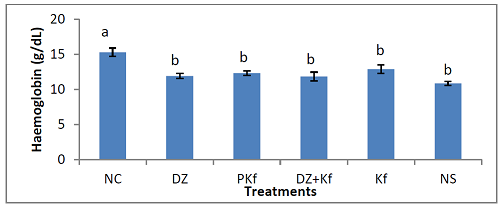
administered normal saline (group VI). Hemoglobin Concentration:
Figure 4
shows the effects of treatments with kaempferol and/or diminazene aceturate on hemoglobin
concentrations in mice infected with T. brucei
brucei. There was significant (P < 0.001) decrease in the mean hemoglobin
concentration in mice pre-treated with kaempferol ( group II) and those that were infected and administered
normal saline (group VI) when compared to mice treated with diminazene
aceturate only (groups III), kaempferol and diminazene aceturate (group IV) and
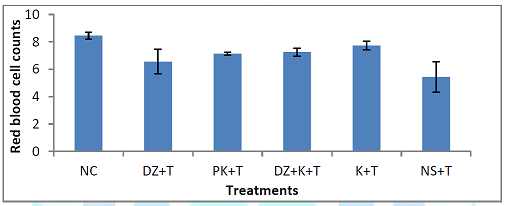
kaempferol only (group V). Red
Blood Cell Count: Figure 5 shows the effect of treatments
with kaempferol and/ or diminazene aceturate on red blood cell count of mice
infected with T. brucei brucei. A
significant (P < 0.001) increase in the mean red blood cell count was
recorded in mice treated with diminazene aceturate only (groups III),
kaempferol and diminazene aceturate (group IV), kaempferol only (group V) when
compared to mice pre-treated with kaempferol ( group II) and those that were infected and administered normal saline (group VI). Effect of the
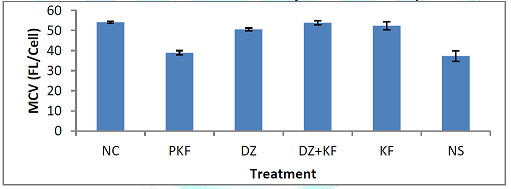
Treatment on Erythrocytic Indices Mean Corpuscular
Volume (MCV): Figure 6 shows the effect of the treatment with kaempferol
and/ or diminazene aceturate on mean corpuscular volume (MCV) of mice infected
with T. brucei brucei. There was
significant (P < 0.001) decrease in the mean values of MCV in mice
pre-treated with kaempferol (group II) and those that were infected and
administered normal saline (group VI) when compared to mice treated with
diminazene aceturate (III), diminazene aceturate and kaempferol (IV) and
kaempferol only (V). Mean
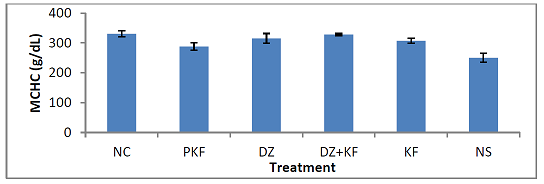
Corpuscular Hemoglobin Concentration (MCHC): Figure 7
shows the effect of the treatments with kaempferol and/ or diminazene aceturate
on mean corpuscular hemoglobin
concentration (MCHC) of mice infected with T.
brucei brucei. There was significant (P < 0.001) decrease in the mean
values of MCHC in mice pre-treated with kaempferol (group II) and those that
were infected and administered normal saline (group VI) when compared to mice
treated with diminazene aceturate (III), diminazene aceturate and kaempferol
(IV) and kaempferol only (V). Mean Corpuscular
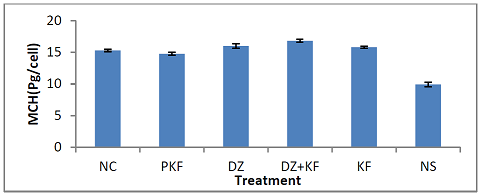
Hemoglobin (MCH): Figure 8 shows the effect of the treatments with kaempferol
and/ or diminazene aceturate on mean corpuscular hemoglobin (MCH) of mice
infected with T. brucei brucei. There
was significant (P < 0.001) decrease in the mean values of MCH in mice
pre-treated with kaempferol (group II) and those that were infected and
administered normal saline (group VI) when compared to mice treated with
diminazene aceturate (III), diminazene aceturate and kaempferol (IV) and
kaempferol only (V). Total White
Blood Cell Count: Figure 9 shows the effects of the treatments with kaempferol
and/ or diminazene aceturate on total white blood cell count of mice infected
with T. brucei brucei. There was
significant (P < 0.001) decrease in the mean total leucocyte count in mice
pre-treated with kaempferol (group II) and those that were infected and
administered normal saline (group VI) when compared to mice treated with diminazene
aceturate only (groups III), diminazene aceturate and kaempferol (group IV) and
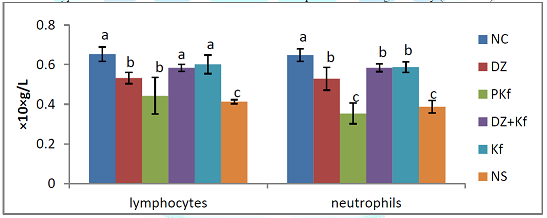
kaempferol only (group V). Effect of
Treatments on Differential Leucocyte Count Figure 10 shows the
effects of treatments with kaempferol and/ or diminazene aceturate on
lymphocyte and neutrophil count of mice infected with T. brucei brucei. Significant (P < 0.05) decrease in the mean
lymphocytes and neutrophil count were observed in mice pre-treated with
kaempferol (group II) and those that were infected and administered normal
saline (group VI) when compared to mice
treated with diminazene aceturate only (groups III), diminazene
aceturate and kaempferol (group IV) and
kaempferol only (group V). The
clinical signs observed were; anorexia, loss of body weight, pale ocular mucous
membrane and weakness and are more obvious in mice pre-treated with kaempferol
(group II) and those that were infected and administered normal saline (group
VI), this agrees with the report of Kobo et al (2014) in rats with experimental
Trypanosoma brucei brucei infection and then treated with two mixture
of flavonoids (hesperidin and Daflon®), severity of these signs depends on the
strain of infecting trypanosomes, dose of the parasite during infection, immune
status of the host and host susceptibility. Variable disorders occur sequel to
trypanosome infection in animals (Adamu et al., 2009), depending on the
virulence of the infecting trypanosome, the infective dose and the immune
status of the host. The symptoms usually associated with trypanosomosis
includes; pallor of the mucous membranes, enlargement of lymph nodes, anorexia
and emaciation (Shimelis et al., 2015). Parasitaemia
was detected four days post-infection, it agrees with the findings of Ibrahim
et al (2016) in mice, rats and rabbits experimentally infected with Trypanosoma brucei brucei. So far,
little has been achieved in terms of understanding the variations in pre-patent
period and course of T. b. brucei infection in various laboratory animal
species because it rapidly divide in the blood stream of their host by binary
fission, resulting in the large population of the parasites within short time
(Ibrahim et al., 2016). Parasitaemia
increased progressively in groups II (pre-treated with kaempferol), V (treated
with kaempferol only) and VI (administered normal saline) up to day nine
post-infection where all mice were sacrificed by severing their jugular veins. There
was significant (P < 0.001) decrease in the level of Parasitaemia in mice
treated with kaempferol only when compared to mice pre-treated with kaempferol
and those that were administered normal saline, this agrees with the findings
of Kobo et al (2014) which showed delayed proliferation of Trypanosoma brucei brucei in rats treated with mixtures of flavonoids
(hesperidin and daflon®), the effect was attributed to scavenging ability of
the flavonoids on free radicals generated during the course of infection. Kaempferol
is also extensively metabolized in the liver to form glucurono-conjugated and
sulfo-conjugated forms (Calderon-Montano et al., 2011), these forms of
kaempferol, and kaempferol itself, can then be excreted in urine. About 2.5% of
kaempferol ingested is excreted as urine; much of the ingested kaempferol is
present in the plasma and tissues in nanomolar concentrations (Calderon-Montano
et al., 2011). In addition, flavonoids may regenerate other antioxidants with
known immune-enhancing activity, such as vitamin E (Zhu et al., 2000) and
carotenoids (Pietta and Simonetti, 1998), therefore, this could be the reason
why kaempferol was effective at reducing the level of parasiataemia in the
infected mice treated with kaempferol. There was an absolute clearance parasite
from the blood stream of mice treated with diminazene aceturates only (group
III) and the combination of diminazene aceturate and kaempferol (group V) one
day after treatment and that the clearance may be due to diminazene (Saba et
al., 2007). The
mean PCV, Hb concentration and RBC count reduced significantly (P< 0.001) in
groups II (pre-treated with kaempferol), V (treated with kaempferol only) and
VI (administered normal saline)
indicating anemia, this agrees with the work of Ukpai and Nwabuko (2014)
who reported the effects of Trypanosoma
brucei brucei on Hematological parameters and pathology of internal organs
in albino rats, Anemia observed was
attributed to mechanical injury to RBC, chemicals produced by live and dead
trypanosomes and also lipid peroxidation. Anemia which is regarded as the most consistent
finding in trypanosomosis of man and domesticated animal has also been reported
in T. vivax infected cattle and goats
(Saror, 1980), T. congolense infected
sheep (Bisalla, 2007), T. congolense infected
dogs (Gow et al., 2007), T. brucei brucei
infected goats, sheep and rabbits (Taiwo et al., 2003; Seed, 1969). The
pathophysiology of Anemia in
trypanosomiasis is complex and multi factorial in origin (Naessens et al.,
2005). It initiates a cascade of events leading to hemolytic Anemia and cardiovascular collapse
(Anosa, 1988). Kaempferol used in this study showed significant level of
protection of the red blood cells in the infected mice treated with kaempferol
only (group V), this may be due to its antioxidant effect and ability to
scavenge the free radicals generated by the parasite during the course of
infection, thereby reducing the free radical loads hence prevent erythrocyte
membrane from oxidative damage (Procházková et al., 2011). Umar et al (2007;
2001) have reported protective effect of vitamin E and C in trypanosomes
induced Anemia. Murray and Dexter
(1988) showed that the severity of Anemia
during trypanosome infection could be related to differences in virulence among
trypanosome strains. Erythrocytic
indices were determined so as to classify Anemia
morphologically (Adamu et al., 2009). There was a significant (p<0.001)
reduction in the mean values of MCV and MCHC in mice pre-treated with
kaempferol (II) and those that were
administered normal saline (group VI), resulting in microcytic
hypochromic Anemia, therefore the
results obtained in this study agrees with that reported by Kobo et al., (2014)
where microcytic hypochromic Anemia
was observed only in untreated rats infected with T. brucei brucei and
disagrees with the finding of Ibrahim et al (2016) where macrocytic hypochromic
Anemia was observed in mice, and rats
with experimental Trypanosoma brucei
brucei infection. Microcytic hypochromic Anemia occur due to erythrogenesis that takes place after the onset
of infection trypanosoma, at which time immature erythrocytes are released into
the systemic circulation (Igbokwe et al.,1994). Microcytic hypochromic anemia
is a blood disorder characterized by small red blood cells (erythrocytes) which
have insufficient hemoglobin and hence have a reduced ability to carry oxygen
through the body (Ford, 2013). The increased MCV and MCHC in kaempferol treated
(group V) may be due to kaempferol
ability to protect red blood cells from oxidative damage by scavenging
free radicals that are detrimental to biomolecules. Mean
WBC, lymphocytes and neutrophils significantly (P < 0.05) decreased in mice
pre-treated with kaempferol (groups II) and those that were administered normal
saline (group VI) agrees with the work of Takeet and Fagbemi (2009) in rabbits
with trypanosomosis, these researchers attributed this reduction to
immunosuppressive actions of trypanosomes. Leucopenia after an initial period of
leucocytosis is a common finding in T. b. brucei infection in laboratory animal
(Ibrahim et al., 2016). Leucopoenia in animal with trypanosomosis has been
reported to be due largely to ineffective or depressed granulopoiesis in the
bone marrow (Anosa et al., 1997a). I
sincerely appreciate laboratory technologists of the Departments of Veterinary
Pharmacology and Toxicology, Veterinary Parasitology and Entomology and
Veterinary Pathology); especially the assistance of S. Musa, A. H. Yau Abdulwahab, A. Sani, D. Otie, Y. Idris, Mal. Yunusa and U. Lawal,
is highly appreciated, who have contributed immensely for the success of this
work. 1.
Adamu S, Barde N, Abenga JN, Useh NM, Ibrahim NDG et al., Experimental Trypanosoma brucei infection-induced
changes in the serum profiles of lipids and cholesterol and the clinical
implications in pigs (2009) J Cell Ani Bio 3: 15-20. Muhammad Y, School of
Agriculture, Department of Animal Health and Production, Binyaminu Usman
Polytechnic, Hadejia Jigawa State, Nigeria, Tel. +2347063249774, E-mail: yamohad@gmail.com Muhammad Y, Suleiman
MM, Jatau IDand Chiroma MA. Effect of Kaempferol, Diminazene
Aceturate and their Combination on Hematological Parameters in Trypanosoma brucei brucei
Experimentally Infected Mice (2018) Pharmacovigil and Pharmacoepi 2: 1-8 Hematology, Kaempferol, Trypanosoma brucei brucei
Effect of Kaempferol, Diminazene Aceturate and their Combination on Hematological Parameters in Trypanosoma brucei brucei Experimentally Infected Mice
Muhammad Y, Suleiman MM, Jatau ID and Chiroma MA
Abstract
Full-Text
Introduction
Materials and
Methods
Results

Discussion
Acknowledgement
References
2.
Anene BM, Onah DN and Nawa Y. Drug resistance in pathogenic African
trypanosomes: what hopes for the future? (2001) J Vet Parasitol 96: 83-100.
3.
Anosa VO, Logan-Henfrey LL and Wells CW. The Hematology of T.congolense infection in cattle 11: Macrophages structure and
function in adult Boran cattle (1997) Int J Comparative Haematol 7: 23-29.
4.
Anosa VO. Hematological and biochemical changes in human and animal
trypanosomosis Parts I & II (1988) Revue d Elevage et de Medicine Veterinaire
des pays Tropicaux 41: 65-78.
5.
Bisalla M, Ibrahim NDG, Lawal IA and Esievo KAN
Serum total protein, albumin and albumin globulin ratio in Yankassa
sheep experimentally infected with Trypanosoma
congolense and immunomodulated with levamizole (2007) J Proto Zol Res 17:39-43.
6.
Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C. and Lopez-Lazaro MA. Review
on the dietary flavonoid kaempferol (2011) Mini-Reviews in Medi Chem 11:
298–344.
7.
Dacie JV and Lewis SM. Practical Hematology 7th Edn (1991) Churchill Livingston,
UK 659-661.
8.
Ford J. Red blood cell morphology (2013) Int J Lab Hematol 35: 351–357.
9.
Gow AG, Simpson JW and Picozzi K. First report of canine Africa trypanosomosis
in the UK (2007) J small ani prac 48: 658-661.
10.
Ibrahim A, Mbaya AW, Anene BM and Luka J. Comparative Parasitaemia and Hematology
of mice, rats and rabbits experimentally infected with Trypanosoma brucei brucei and their responses to diminazene
diaceturate (Veriben®) therapy (2016) Asian Pacific J Trop Disease 6: 527-532.
11.
Igbokwe IO. Dyserythropoiesis in animal trypanosomosis (1989) Trop Veterinarian
42: 423-429.
12.
Igbokwe IO. Mechanism of cellular injury in African Trypanosomiasis (1994)
Annals Trop Medi Parasitol 64: 611–615.
13.
Kinabo LDB. Pharmacology of existing drugs for Animal Trypanosomiasis (1993) Acta Tropica 54: 169 -183.
14.
Kobo PI, Ayo JO, Tagang A, Zezi AU and Maikai VA.. Hematological Changes in Trypanosoma brucei brucei Infected
Wistar Rats Treated with a Flavonoid Mixture and/or Diminazene Aceturate (2014).
Bio Medi 6: 3
15.
Leach TM, and Roberts CJ. Present status of chemotherapy and chemoprophylaxis
of animal trypanosomiasis in the eastern hemisphere (1981) J Pharma and
Therapeutics 13: 91–147.
16.
Maikai VA, Salka MN, Adeiza AA and Makeri HK. Assessment of Isometamidium
chloride and diminazene aceturate in laboratory mice infected with field
isolates of T. congolense from
naturally infectedcattle (2007) J Prodn Agri and Tech 3: 147 – 152.
17.
McDermott JJ, Sidibe I, Bauer Diarra B, Clausen PH, Woitang, T, Ouedraogo D et
al. Field studies on the development and impact of drug resistance animal
trypanosomes in market oriented production systems in the southern Guinea Zone
of West Africa (2000). News letter N0.2 of EU.
18.
Morrison WI, Max Murra, Sayer PD and Preston JM. The pathogenesis of
experimentally induced Trypanosoma brucei infection in the dog, Tissue and
organ damage (1981) Am J Pathol 102: 168-181.
19.
MMurray M and Morrison WI. Parasitaemia and host susceptibility in African
trypanosomiasis (1978) Proceedings of a workshop, Nairobi, Kenya 71-81.
20.
Naessens J, Kitani H, Yagi,Y, Sekikawa K.and Iraqqi F. TNF-a mediates the
development of Anemia in a murine
Trypanosoma brucei rhodesiense infection, but not the Anemia associated with a murine T.
congolense infection (2005) Clin Exp Immunol 139: 403-410.
21.
Ndungu JM, Murrilla GA, Mdachi RM, Mbwambo H, Sinyangwe L et al. Area-wide
appraisal of drug resistance in trypanosomes infecting cattle in East and South
Africa (1999) In: Proceedings of the International Scientific council for Trypanosomiasis
Research and Control 25th meeting Mombasa, Kenya.
22.
Procházková DI, Boušová I and Wilhelmová N. Antioxidant and prooxidant
properties of flavonoids (2011) Fitote 82: 513–523.
23.
Saba AB, Adedapo AA, Ayagbemi AT and Odudu ZK. Laboratory evaluation of
sensitive pair of diminazene aceturate and isometamedium chloride as
combination therapy for animal trypanomosis (2007) Folia Vet 51: 169-174.
24.
Saror DI. Observation on the course and pathology of Trypanosoma vivax in red
Sokoto goats (1980) Res Vet Sci 28: 36-38.
25.
Saror DI. Aspects of the Anemia of
acute bovine trypanosomiasis Proceedings of the first National Conference on
Tsetse and Trypanosomiasis (1982) Research Kaduna, Nigeria 12-14.
26.
Schalm OW, Jain NC and Carroll EJ. Veterinary Hematology 3rd Edn (1975) Lea
& Febiger, USA 369-386.
27.
Seed JR. Trypanosoma gambiense and Trypanosoma lewisi: Increase vascular
permeability and skin lesion in rabbits (1969) Exp Parasitol 26: 214-223.
28.
Shehu SA, Ibrahim NDG, Esievo KAN and Mohammed G. Role of erythrocyte surface
sialic acid inducing Anemia in
Savannah Brown bucks experimentally infected with Trypanosoma evansi (2006) Vet
Arhive 26: 521-530.
29.
Shimelis D, Melkamu B, Getachew T, Getachew A, David, B et al. Comparative
clinico-Hematological analysis in young Zebu cattle experimentally infected
with Trypanosoma vivax isolates from tsetse infested and non-tsetse infested
areas of Northwest Ethiopia (2015) Acta Veterinaria Scandinavica 57:24.
30.
Taiwo VO, Olaniyi MO and Ogunsanmi AO. Comparative plasma biochemical changes
and susceptibility of erythrocytes to in vitro peroxidation during experimental
Trypanosoma congolense and Trypanosoma brucei infection in sheep (2003) Israel J
Vet Medi 58: 435-443.
31.
Takeet MI and Fagbemi BO. Hematological, Pathological and Plasma biochemical
Changes in rabbits experimentally infected with trypanosoma congolense (2009) Sci
World J 4: 2.
32.
Ukpai OM and Nwabuko OP. Effects on Hematological parameters and pathology of
internal organs of Trypanosoma brucei
brucei infected albino rats (2014) Nigerian J Biotech 27: 8-13.
33.
Umar IA, Igbalajobi FI, Toh ZA, Gidado A, Shugaba A et al. Effects of oral
administration of repeated doses of vitamin E on some biochemical indices in
rats infected with Trypanosoma brucei
brucei (2007) West African J Bio Sci 12: 1–7.
34.
Zhu QY, Huang Y and Chen ZY. Interaction
between flavonoids and alphatocopherol in human low density lipoprotein (2000)
J Nutritional Biochem 11: 14-21.*Corresponding author
Citation
Keywords