Review Article :
Background:
The
polymorphism of cytochrome P450 2C19 (CYP2C19) has been documented as the
determinant variability in the antiplatelet effect of clopidogrel. The relation
between CYP2C19 polymorphism
and the antiplatelet efficacy of clopidogrel in Indonesian patients with coronary
artery disease (CAD) is unknown. To address this issue, we examined the
distribution of CYP2C19 genotypes and platelet aggregation, and assessed the
impact of CYP2C19 polymorphism on response to clopidogrel and cardiovascular
events. Methods:
This observational analytic study with prospective cohort approach was
conducted in Wahidin Sudirohusodo and Hasanuddin University Hospital, Makassar.
We measured the CYP2C19 genotype by polymerase chain reaction-restriction
fragment linked polymorphism (PCR-RFLP) method and platelet aggregation by optical platelet aggregometry with 10 μmol of adenosine
diphosphate (ADP) in 69 patients with stable CAD who were treated with
clopidogrel. Platelet hyperaggregation was defined as maximal platelet
aggregation > 94.3%. The patients were followed up every month at the
outpatient department for 6 months or at end point. The end point was acute
myocardial infarction, ischemic stroke, or cardiovascular death. Results:
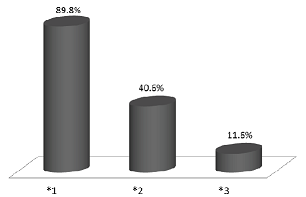
Distribution of CYP2C19 alleles were 89.8%, 40.6%, and 11.6%, in CYP2C19*1,
CYP2C19*2, and CYP2C19*3, respectively. Distribution of CYP2C19 genotype were
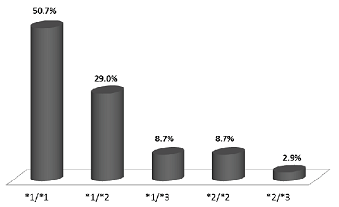
50.7%, 29.0%, 8.7%, 8.7%, and 2.9% in CYP2C19*1/*1, *1/*2, *1/*3, *2/*2, and
*2/*3, respectively. Platelet hyper aggregation was more in patients with
polymorphism than wild type (p 0.034;
OR 3.707) and was associated with cardiovascular events (p 0.030; OR 13.250). There was acute
myocardial infarction in 2 patients, ischemic stroke in 1 patient, and cardiovascular
death in 1 patient. All of these patients were carrying at least one variant
allele of CYP2C19; details of genotype were in two patients with CYP2C19*1/*2,
one patient with *2/*2, and one with *2/*3 alleles. Conclusion:
CYP2C19*2 and *3 were associated with cardiovascular events due to platelet
hyper aggregation. Cardiovascular diseases (CVDs) are the number one cause
of death globally; exceeding other diseases. An estimation of 17.3 million
people died from CVDs in 2008, representing 30% of all global death [1].Of
these, an estimated 7.3 million were due to coronary artery disease (CAD) [2]. Reperfusion of the coronary arteries is the first-line treatment
in ischemic heart disease. The treatment methods are the administration of
fibrinolytic drugs, percutaneous coronary intervention (PCI), or coronary
artery bypass surgery (CABG). Indeed, PCI is the treatment of choice for acute
myocardial infarction (MI), if it is done on time [3,4]. However, stent thrombosis can occur after stent
placement. Many studies have been conducted on the prevention of stent
thrombosis with antiplatelet therapy. The American Society of Cardiology (ASC)
guideline has recommended the administration of clopidogrel, in combination
with aspirin, in patients using bare metal stent (BMS) for at least one month
and up to 12 months in recent studies [5] and in patients using drug-eluting stent
(DES) for at least 12 months [6]. Clopidogrel is a
pro-drug agent that, after becoming an active metabolite, selectively blocks
ADP dependent platelet activation and aggregation. The drug requires the enzyme
cytochrome P450 2C19 (CYP2C19) function for its activation and antiplatelet
effect. In some patients, clopidogrel has no antiplatelet effect or its effect
is reduced. The responsiveness to clopidogrel is determined by genetic and
acquired factors, and is one of the important factors in stent thrombosis and
cardiac events after the stent placement in patients with CAD.7
Single nucleotide polymorphism of CYP2C19 that reduce the activity of this
enzyme are among the causes of racial differences in response to the
antiplatelet effect of clopidogrel [7]. The presence of CYP2C19*2 allele decreases
the response to antiplatelet effects of clopidogrel [8]. To
date, the effect of the CYP2C19 polymorphism on antiplatelet effect of
clopidogrel and cardiovascular events in Indonesian patients with CAD remains
unknown. Methods
We conducted
prospective cohort study in Wahidin Sudirohusodo and Hasanuddin University
Hospital, Makassar, South Sulawesi, Indonesia from September 2013 until
September 2014. Ethical approval
was obtained from the ethics committee of Hasanuddin University and informed
consent was obtained from all participants. We studied 69 patients
of angiographically proven CAD taking clopidogrel. Patient with acute coronary syndrome
received anticoagulant, or antiplatelet agents other than clopidogrel or
aspirin were excluded. Genotyping
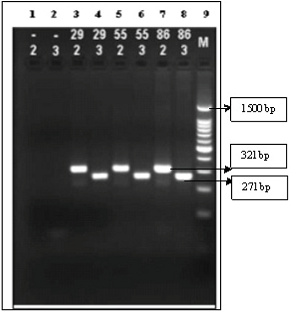
Table 1: The PCR amplification for CYP2C19*2 and CY2C19*3. PCR
product was detected by electrophoresis method with agarose gel 2% and Ethidium
Bromide staining. Bands were detected by a short wavelength UV transluminator
with Gel Doc (BioRad). The results were band 321 bp for CYP2C19*2 and 271 bp
for CYP2C19*3. The example of electrophoresis gel result is shown in Figure 1. Digestion of the
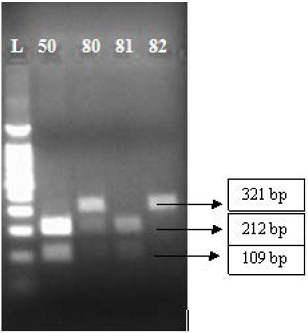
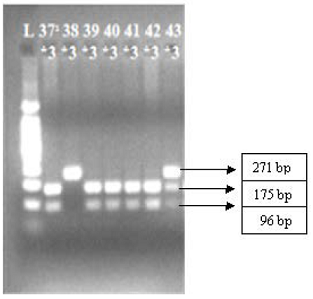
CYP2C19*2 amplicon with SmaI resulted
in products of 212 and 109 bp (homozygous wild type; c.681 G/G); 321, 212 and
109 bp (heterozygote; G/A); and a single undigested product of 321 bp
(homozygous *2; A/A). Digestion of the CYP2C19*3 amplicon with BamHI resulted in products of 175 and 96
bp (homozygous wild-type; c.636 G/G); products of 271, 175 and 96 bp
(heterozygote *3; G/A); and a single undigested product of 271 bp (homozygous
*3; A/A), as shown in figures 2 and 3. Platelet aggregation was measured by optical platelet aggregometry with AggRam platelet
aggregometer; Helena Biosciences Europe). This procedure is performed on a
turbidimetric aggregometer, as first described by Born [10]. The change in absorbance is recorded as platelet rich plasma
is stirred in a cuvette with aggregating reagents added. We used ADP as
aggregating reagent with concentration 10 µM. The platelet aggregation
reference value was 66.9 – 94.3%. Platelet hyperaggregation was defined as as
maximal platelet aggregation more than 94.3%. The subject s were divided into
two groups, (1) hyperaggregation group (n
= 15) and (2) non-hyperaggregation group (n
= 54). The patients were followed up every
month at the outpatient department for 6 months or at end point. The end point
was acute myocardial infarction, ischemic stroke, or cardiovascular death. Continuous variables are expressed as means ± SD. Categorical variables are expressed as
frequencies and percentages. Differences in classifications between two or more
groups were evaluated using Fisher´s exact test or likelihood ratio. Statistical significance was p < 0.05.
All statistical analyses were performed using SPSS 18 for Windows. Clinical characteristics of
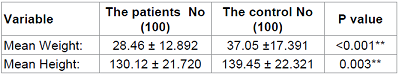
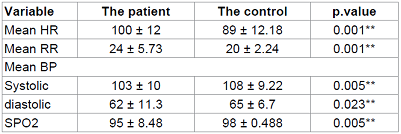
each group (hyper aggregation and non-hyper aggregation) are shown in Table 2. There were no significant
differences in baseline characteristics between hyper aggregation and non-hyper
aggregation groups. Distribution of
CYP2C19 alleles were 89.8%, 40.6%, and 11.6%, in CYP2C19*1, CYP2C19*2, and
CYP2C19*3, respectively as shown in figure 4. Distribution
of CYP2C19 genotype were 50.7%, 29.0%, 8.7%, 8.7%, and 2.9% in CYP2C19*1/*1,
*1/*2, *1/*3, *2/*2, and *2/*3, respectively as shown in figure 5. CYP2C19
Polymorphism and Platelet Aggregation There was more platelet
hyper aggregation in patients with polymorphism than wild type (p 0.034; OR 3.707) as shown in table 3. Cardiovascular events in patient within
study population were: stroke, 1 patient; acute myocardial infarction, 2;
ischemic stroke, 1 patient (Table 4). All of
these patients were carrying at least one variant allele of CYP2C19; details of
genotype were in two patients with CYP2C19*1/*2, one patient with *2/*2, and
one patient with *2/*3 alleles (Table 5). Platelet
Aggregation and Cardiovascular Events
Hyperaggregation
patients were associated with cardiovascular events than non-hyper aggregation
group (p 0.030; OR 13.250) as shown
in table 6. Our study found that CYP2C19*1 was most common than CYP2C19*2 and CYP2C19*3.
Scott et al [11]have reported about frequencies
of CYP2C19 alleles in African, American,
European, East Asian, and South/Central Asiasn population; they found the similar result that CYP2C19*1 allele was most
common than other alleles. The other study in CAD patients also showed that CYP2C19*1 allele was
most common than other allele. Tiong et al [12]showed that in 237
clopidogrel-treated patients among Malaysian multiethnic population, 63.0% were CYP2C19 *1, 29.0% were CYP2C19*2, 6.0% were CYP2C19*3, and 2% were CYP2C19*17. Study of Yamamoto et al in 246 CAD patients showed
the frequency of CYP2C19*1, *2, and *3 were 58.9%, 30.9%, and 10.2% respectively. There are several factors that can influence platelet aggregation. Age,
smoking, diabetes, dyslipidemia, and ASA use are known as factors that can
influence the platelet aggregation. Previous studies have reported that
platelet activation increases with age. For example, Bastyr and colleagues
demonstrated increased platelet phospholipid content, suggesting increases in
transmembrane signalling with age [13]. It has also been shown that
age is associated with an increase in platelet aggregability [14]. Kalliakmanis et al [15] reported that nicotine can inhibit
vascular prostacyclin (PGI2). Prostacyclin is a prostaglandin member that can inhibit
platelet aggregation. In diabetes patients, synthesis of PGI2 is decreased while prostaglandin E-like material is
increased. Furthermore it will increase the synthesis f thromboxane and
stimulate the platelet aggregation [16]. Hypercholesterolemia
can enhance the ability of platelets to aggregate. Acetyl salicylic acid
(ASA) can inhibit the formation of thromboxane
A2 in platelets,
producing an inhibitory effect on platelet
aggregation [17]. Likelihood
ratio was performed to analyzed relationship between hyperaggregation and
non-hyperaggregation group with some variables that can influence the platelet
aggregation. The result was no significant differences between two groups as
shown in table 2. Table 2: Baseline Characteristics of Study Patients. Figure 4: Distribution of CYP2C19 Alleles. Figure 5: Distribution of CYP2C19 Genotype. Clopidogrel is converted to an active
thiol by the cytochrome P450 CYP 3A4 and 2C19 enzymes. Statins that metabolized
by CYP3A4 suggested can attenuate the anti-aggregatory effect of clopidogrel.
Analysis of relationship between concomitant use of statin and clopidogrel in
this study showed that no significant differences between patient with- and
without statin (p 0.791, table 2). This
result showed that concomitant use of statin and clopidogrel did not influence
the platelet antiaggregation effect of clopidogrel. Similar result have been
found by Polena et al [18] that showed
concomitant statins with clopidogrel therapy did not influence the effect of
clopidogrel in platelet aggregation inhibition. We also found that there were no
significant differences between patient with- and without PPI use (Table 2). Competition with PPI with shared metabolization by CYP2C19
may diminish antiplatelet function of clopidogrel. Attenuating
effects on platelet response to clopidogrel have been reported solely for the
PPI omeprazole. Sibbing et al [19] showed that
concomitant use of clopidogrel and omeprazole associated with higher platelet
aggregation compared than patient without omeprazole. But a meta-analysis
showed that there were conflicting and inconsistent data on the interaction
between clopidogrel
and proton-pump inhibitors.
The data were pooled and analysed by study design, but the substantial
statistical, clinical, and methodological heterogeneity mean that it might not
have been appropriate to pool the data [20]. The present study showed that patients
which carrying at least one variant allele of CYP2C19 associated with 3-fold
increased risk for platelet
hyperaggregation (Table 3). Mega et al [21] studied 162 healthy subjects that included from six
studies involving clopidogrel treatment. Polymorphic CYP enzymes tested –2C19,
2C9, 2B6, 3A5, and 1A2. The result showed that carriers of at least one CYP2C19
reduced-function allele had a relative reduction of 32.4% in plasma exposure to
the active metabolite as compared to noncarriers (p < 0.001). In addition, carriers of at least one CYP2C19
reduced-function allele had an absolute difference in reduction of maximal
platelet aggregation (∆MPA) in response to clopidogrel that was 9% less than
noncarriers (p < 0.001), relative
risk reduction of 25%. Trenk et al [22] investigated whether
the loss of function CYP2C19 681G>A *2 polymorphism is associated with high
(> 14%) residual platelet aggregation (RPA) on clopidogrel and whether high
on-clopidogrel RPA impacts clinical outcome after elective coronary stent
placement. They found that between *2 carriers and wild-type homozygotes, there
was significant (p < 0.001) differences in the proportion of patients with
RPA > 14%, both after loading (62.4% vs. 43.4%) and at pre-discharge (41.3%
vs. 22.5%). Our study also showed that patients with
platelet hyperaggregation associated with 13-fold increased risk for cardiovascular events (Table 6). Sofi et al [23]
studied 4564 patients with stable angina,
chronic CAD, or ACS (meta-analysis) and showed that clopidogrel non-responsiveness associated with an increased
risk of recurrent cardiovascular events (OR 3.58; 95% CI, 2.54-5.05 (p < 0.00001) after adjustment for
heterogeneity. Table 3: Relation between CYP2C19 Polymorphism and Platelet Aggregation. Table 4: Cardiovascular Events in Study Population. Table 5: Details of Cardiovascular Events in Study Population. Table 6: Relationship between Platelet Aggregation and Cardiovascular Events. In this study we found that
CYP2C19 polymorphism is associated with cardiovascular events. All of
cardiovascular events occurred in patients which carrying at least one variant
allele of CYP2C19 (Tables 4 and 5). Singh et al [24] showed that CYP2C19*2
polymorphism was associated with higher risk of major adverse cardiovascular
events [RR: 1.28, CI: 1.06-1.54; p = 0.009], cardiovascular death [RR: 3.21,
CI: 1.65-6.23; p = 0.001], myocardial infarction [RR: 1.36, CI: 1.12-1.65; p =
0.002], stent thrombosis [RR: 2.41, CI: 1.69-3.41; p < 0.001]. Mega et al21
showed that carriers of a reduced-function CYP2C19allele have
significantly lower levels of the active metabolite of clopidogrel, diminished
platelet inhibition, and a higher rate of major adverse cardiovascular events,
including stent thrombosis. They found that 395 subjects carrying at least one
CYP2C19reduced-function allele were at higher risk for the primary
endpoint (composite death from cardiovascular causes, myocardial infarction, or
stroke) 12.1% vs. 8.0%; HR for carriers 1.53; 95% CI, 1.07 to 2.19 (p = 0.01). In the present study, we do not measure
plasma concentrations of the active metabolite of clopidogrel, thus, we cannot
provide direct evidence of reduced antiplatelet efficacy of clopidogrel in
patients carrying at least one CYP2C19*2 or *3 variant allele. In addition, we
cannot exclude the effect of other drug metabolism enzymes, such as CYP1A2,
2B6, 3A, and 2C9, on clopidogrel response, besides CYP2C19. Thus, further study
using larger samples are needed in the future. CYP2C19*2 and *3
were associated with cardiovascular events due to platelet hyper aggregation. References Paskalis Indra, Department of Cardiology and Vascular Medicine, Faculty of Medicine, Hasanuddin University, Makassar-Indonesia, Tel: +62 81283651490, E-mail: paskalis.indra@gmail.com Amir M, Mappiare M, Indra P (2017) The Impact of Cytochrome P450 2C19 Polymorphism on Cardiovascular Events in Indonesian Patients with Coronary Artery Disease. CCCM. 1: 18-24. Polymorphism, CYP2C19, Clopidogrel, Coronary artery disease, Indonesia.The Impact of Cytochrome P450 2C19 Polymorphism on Cardiovascular Events in Indonesian Patients with Coronary Artery Disease
Muzakkir Amir, Mirnawati Mappiare, Paskalis Indra
Abstract
Full-Text
Introduction
Study
Population
Genotyping of CYP2C19 alleles (CYP2C19*1, CYP2C19*2, and CYP2C19*3 alleles) was
carried out by polymerase chain reaction-restriction fragment linked
polymorphism (PCR-RFLP) technique [9]. Reaction
mixture for PCR with 30 μL KAPA Taq DNA
Polymerase that consist of 0.6 U
Taq Polymerase, dNTPs 0.1 mM, in PCR Buffer 1X (500 mM Tris/HCl pH 8.3, 100 mM
KCl, 50 mM (NH4)2SO4,), and 1.5 mM MgCl2,
added with 0.4 μM forward and reverse primer, and 6 μL DNA template (DNA
extract). There were 2
master mix for each sample, that consist of primer set *2 and *3. The PCR amplification
was performed with Thermal Cycler Verity (Applied Biosystem) (Table 1).
Platelet
Aggregation Measurement
Clinical
Outcomes
Statistical
Analysis
Results
Patient characteristics
CYP2C19 Alleles and Genotype
CYP2C19
Polymorphism and Cardiovascular Events
Discussion

Limitations
Conclusion
*Corresponding author:
Citation:
Keywords