Research Article :
Zouhra Doukkali*, Mohamed Amine Boumahdi, EL Houcine Bouidida, Khalid Taghzouti Anxiety is considered a
common emotional phenomenon in the human population,occurring in response to physiological
and/or environmentalfactors [1]. When anxiety becomes excessive, it may be considered as an
anxiety disorder, and can critically decrease the quality of life inducing several
psychosomatic diseases. Benzodiazepines are the major class of compounds used
in anxiety and they have remained the most commonly prescribed treatment for
anxiety [2]. Benzodiazepines can lead to disturbing effects, such as amnesia, dependence liability,
and sedation [3,4]. Therefore, the development of other anxiolytic drugs
without such adverse effects is important for the treatment of anxiety
disorders. Several traditionally used plants exhibit pharmacological properties
with great potential for therapeutic applications in the treatment of central
nervous system disorders, such as anxiety disorders [5]. In folk medicine, some
species belonging to the family Euphorbiaceae, such as Mercurialis annua,
is known to possess anxiolytic action [6]. Mercurialis annua L.
(Euphorbiaceae) is a wind-pollinated annual plant that occupies ruderal
and roadside habitats throughout central and western Europe and around the
Mediterranean Basin [7]. The species is a winter annual in the Mediterranean
region, and has long been known to have tranquillizing effects among the
Moroccan people [6,8]. Reaching 10–50 cm in height, M. annua contains
large amounts of flavonoids [9] and of the pyridinone-type alkaloid, hermidin
[9]. Ethnobotanical reports attribute purgative, diuretic or antisyphilitic effects
to the dried plant. Despite the wide spread use of M.annua as an
anxiolytic, thereare no
pharmacological data to support such effect, therefore, we explored the
anxiolytic-like effects of Methanolic extract of Ma by using the hole board
test, the light–dark box test, and the rota rod test,
classic experimental models. Animals Plant material Preparation of
the methanolic extract Drugs and
chemicals Treatment
schedule Behavioral
paradigms Hole Board test:
The
hole board test [11] was adopted in this test. It is made of gray Perspex.
The LETICA board (signo 720; Printer LE 3333) of dimensions 40 cm X 40 cm,
contained 16 evenly spaced holes (3 cm diameter and 2.2 cm depth), with inbuilt
infra-red sensors was used for the study. The matt finishing of the upper panel
avoids reflections which may alter the animal behavior. An animal was placed in
the center of the hole board and allowed to freely explore the apparatus for 5
min. The number of times an animal dipped its head into the holes was
automatically counted and recorded by the instrument [12]. Rota rod test: The effect on motor
coordination was assessed using a rota-rod apparatus (LE 8500). Rota rod
consisted of a base plant form and aniron rod of 3 cm diameter and 30 cm
length, with a non-slippery surface. Th rod was divided into four equal
sections by three disks. The animals were pre-selected in a training session 24
h before the test, based on their ability to remain on the bar (at 12 rpm) for
2 min, and then allowing four mice to walk on the rod at the speed of 12 rpm at
the same time observed over a period of 30, 60, and 90 min. Intervals between
the mounting of the animal on the rotating bar and falling off of it were
registered automatically as the performance time. Time spent in the apparatus
was observed for 5 min duration (300 s). Apparatus was cleaned thoroughly between
trials with water. All behavioral recordings were carried out with the observer
blind to the treatment the mice had received. Statistical
analysis Ethics approval Light/Dark test Hole board test Rotarod test The
pathophysiologic mechanisms associated with anxiety disorders are very complex.
Dysregulation of the GABAergique, serotoninergic, dopaminergic and adrenergic neurosystems have all
been implicated in the pathophysiology
of anxiety [13]. Benzodiazepines are the major class of compounds used in anxiety
and they have remained the most commonly prescribed treatment for anxiety [2].
BZDs produce their pharmacological actions via specific high affinity binding
sites on a supra molecular complex composed of GABA-A and a BZD receptor
coupled with a chloride ion channel. Other anti-anxiety medications include test
and the hole board, and to examine motor coordination we used Rota rod test.
Furthermore, the effects of Mercurialis annua and diazepam on these
animal models were compared to determine whether the behavioral profile of M.annua
differed from an established anxiolytic drug. The light/dark test is based on
the innate aversion of rodents to brightly illuminated areas and on the
spontaneous exploratory behaviour of rodents in response to mild stressors,
that is, novel environment and
light [15]. Thus, in the light/dark test, drug-induced increase in behaviours in
the white part of the box, in which white compartment is illuminated and black
compartment is darkened, is suggested as an index of anxiolytic activity. An
increase in transitions is considered to reflect anxiolytic activity. Our results
showed that the extract (100 mg/kg) increased time spent in the light chamber,
suggesting anxiolytic action of M.annua. The data
presented hereby reinforce the traditional use of Mercurialis annua by
Moroccan people to treat anxiety [6]. Despite the wide spread traditional use
of Mercurialis annua for treating various disorders there are no reports
of scientific evaluation of its anxiolytic activity. Our study shows that the Mercurialis
annua extract had marked effects on the anxiety-related behavioural
parameters on exposure to the light/dark test and the hole board in mice. Mercurialis
annua extract
causes an “anxiolytic”
behavior comparable with the effects of diazepam. Future studies will be focused
on the neurobiological mechanisms of action and possible interactions of Mercurialis
annua with classical neurotransmitters
and the phytoconstituent(s) responsible for the observed central effects has to
be isolated and identified. All authors
assert that none has any commercial or financial involvements that might
present an appearance of a conflict of interest in connection with the
submitted manuscript. DZ, I carried
out all the studies and drafted the manuscript with the help of the above authors,
as regards TK participated in this work and drafted with me the manuscript. EHB
helped us in the chemistry part and NM carried out the behavioral tests with me,
MAB helps me in the behavioral tests,and CY is the director of the laboratory
he advises me and guides me always in my work, after my PhD supervisor KA she
corrects the manuscript, guides me and advises me. All authors read and
approved the final manuscript. We are grateful
to Pr.ZELLOU Amina, PES in faculty of medicine and pharmacy .Rabat, Thanks are
due to Pr. Halim Khammar and Pr. Fennane, botanists of
scientific institute for their participation on this work, we would also like
to thank the students PhD: Meriem Jemly, Rabie Kamal, Hanae Hosni, Amina
Bounihi for their encouragement and interest in the research work. 1. Larissa
Fernanda de A. Vieira, Maria Danielma dos S. Reis. Anxiolytic-like effect of
the extract from Bowdichia virgilioides in mice. Revista Brasileira de Farmacognosia.
Brazilian Journal of Pharmacognosy (2013); 23(4): 680-686.Neuropharmacological Evaluation of Methanolic Extract from Mercurialis Annua a Plant used in Moroccan Traditional Medicine
Abstract
Current
therapeutic for the treatment of anxiety is associated with a wild variety of prominent
side effects. The traditional use of plant extract to health care can indicate
an important source of new pharmaceuticals. In Morocco traditional medicine,
the use of Mercurialis annua is commonly recommended for relief of
anxiety. Nevertheless, despite its popular use there are no studies related to
its possible neuropharmacological effect. Here, we investigated the possible
anxiolytic effect of the extract of M.annua after acute treatment in
mice. The methanolic extract from the aerial parts of M.annua (100, 200
or 400 mg/kg) was orally administered, and its anxiolytic effect was evaluated
in hole board test, the light–dark box test, and motor coordination with the
rota rod test. Diazepam was employed as standard drug 1mg/kg. The methanolic
extract of Ma 100 mg/kg increased the time spent in the brightly-lit chamber of
the light/dark box, as well as in the number of times the animal crossed from one
compartment to the other. Performance on the rota rod was unaffected. In the
hole board test, the extract significantly increased head-dip counts. These
results provides support for anxiolytic activity of Mercurialis annua,
in line with its medicinal traditional use, and may also suggest a better
side-effect profile of Mercurialis annua relative to diazepam.
Full-Text
Introduction
Materials and
Methods
Balb/c mice of
either sex (20-30g) were employed in the present study. Animals were procured
from the animal experimental center of Mohammed V. University, Medecine and pharmacy Faculty, Rabat.
Animals were provided normal diet and water ad libitum and were maintained in a
room with controlled temperature
of 20-25°C, and lighting (light/dark 12:12 hour) in polypropylene cages. The
animals were acclimatized to the laboratory condition before experiments at
least 1h. The animals were kept fasted 2h before drug administration, the Open
field and the elevated plus maze were performed between 2p.m and 6p.m.
The aerial part
of Mercurialis annua was collected from the north of Morocco near the
town of Wazzan (Jaaouna el Basra), with assistance of a traditional medical
practitioner. The plant was authenticated by botanists of scientific institute
Pr. M. Ibn Tatou and Pr. Halim Khammar. A voucher specimen (N° RAB78984) was
deposited in the Herbarium of Botany Department of the Scientific Institute of
Rabat.
The aerial part
was dried at room temperature and crushed.700 g of plant material was extracted
with six liter of methanol by maceration at room temperature (25°C) over period
of 48 hours. Methanol containing the extract was then filtered through Whatman paper and the
solvent was vacuum-distilled at 60 °C in a rotary evaporator. The remaining
extract was finally dried by desiccator.
Final extract was a dark green paste, with 21.22 % dry weight. The residue was
dissolved in water for final suitable concentrations.
The methanolic
extract of Mercurialis annua was suspended in distilled water. Diazepam
was diluted with saline to the required concentration before use. It is well
known that benzodiazepines
act as anxiolytics at low doses and that they induce sedation and muscle
relaxant effects at higher doses [10]. Therefore, we used diazepam
(1mg/kg; ip) as a positive control for anxiolytic-like effects.
Animals were divided
into five groups, each consisting of six mice. Experimental groups of mice were
treated orally (p. o.) with methanolic extract of Mercurialis annua at
doses of (100, 200 and 400 mg/kg),
whereas control groups received normal saline by the same routes, the trial was
carried out 1H after the treatments. Diazepam (1 mg/kg) was administered
intraperitoneally (i.p.) the trial was carried out 30 min after the treatments.
All drugs were freshly prepared before each experiment. The doses of extracts were
calculated to administer 0.25 ml of the suspension of extracts to the mice of
20 g. The anxiolytic activity was examined by using the hole board test, the
light–dark box test, and the rota rod test.
Light/Dark test:
The
apparatus consisted of two 20 cm X 10 cm X 14 cm plastic boxes: one light
compartment painted white and brightly illuminated and the other was dark
painted black and dimly illuminated with red light. The mice were allowed to
move from one box to the other through an open door between the two boxes. The
illumination in the black compartment was 50 lux, in the white area it was
increased to 1000 lux, generated by an extra light source. A mouse was put into
the light box facing the hole. The transition between the light and the dark
box and time spent in the light box were recorded for 5 min.
All the results
were expressed as mean ± SEM. All statistical analysis was done using one way
analysis of variance (ANOVAfollowed by the Tukeys post hoc test. P<0.05 was
considered as significant when compared to their respective control group.
The study was
conducted in accordance with the accepted principles outlined in the “Guide for
the Care and Use of Laboratory
Animals” prepared by the National Academy of Sciences and published by the National
Institutes of Health and all efforts were made to minimize animal suffering and
the number of animals used. Ethics approval was obtained from the central laboratory
of animal of Facutly of medecine and pharmacy from the Mohammed V
University of Rabat.Results
Mercurialis annua at the dose of
100 mg/kg and diazepam (1 mg/kg) induced a significant increment of number of
transition and time spent by mice on the illuminated side of the apparatus compared
to the respective control group (P <0.001) (Table A).
The dose 100
mg/kg of the plant extract significantly increased the number of head dippings
as compared to control animals (P< 0.01) (Table B).
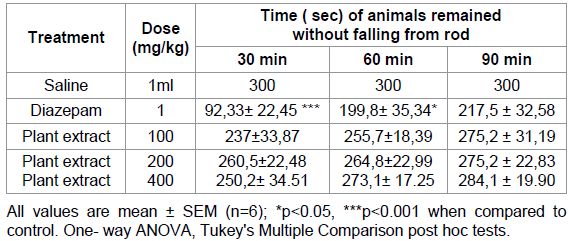
The data shows
that on average the mice treated with 100, 200 and 400 mg/kg p.o. of the
methanolic extract of Mercurialis annua were able to maintain
equilibrium on the rotating rod and stayed on longer without falling (Table C),
whereas diazepam (at 1 mg/ kg only) showed a significant decrease in the locomotor score when
compared to other groups.Discussion
The hole board
test is useful for modeling anxiety in animals, in this test an anxiolytic-like
state may be reflected by an increase in head –dipping behaviors [16,17]. Our
results showed that methanolic extract (100 mg/kg) of Mercurialis annua increased
the head dipping corroborating the anxiolytic-like effect previously shown in
the light- dark test. Rota rod test a classical animal model used to evaluate peripheral
neuromuscular blockade
and the motor coordination [18], a deficit in motor coordination would very
likely affect performance in the behavioral tests. Our findings showed that Mercurialis
annua (100-200 mg/kg), unlike diazepam (1 mg/kg), had no significant effect
on motor coordination. Furthermore, the extract didnt affect motor coordination,
is additional evidence of centrally mediated actions and not blockade of
neuromuscular system [19,20]. The M.annua extract showed promising
anxiolytic effects without causing any neuromuscular side effects.

Conclusions
Conflict of
Interest Statement
Authors
Contributions
Acknowledgment
References
2. Lader M,
Morton S. Benzodiazepine problems. Br J Addict (1991) 86: 823-828.
3. Czobor P,
Skolnick P, Beer B, Lippa A. A multicenter, placebo-controlled, double-blind,
randomized study of efficacy and safety of ocinaplon (DOV 273,547) in
generalized anxiety disorder. CNS Neurosci Ther (2010)16: 63-75.
4. Emamghoreishi
M, Khasaki M, Aazam MF. Coriandrum sativum: evaluation of its anxiolytic
effect in the elevated plus-maze. J Ethnopharmacol (2005) 96: 365-370.
5. Faustino TT,
Almeida RB, Andreatini R. Plantas medicinais no tratamento do transtorno de
ansiedade generalizada: uma revisão dos estudos clínicos controlados (Medicinal
plants for the treatment of generalized anxiety disorder: a review of
controlled clinical studies). Rev Bras Psiquiatr. (2010) 32: 429-436.
6. Z. Doukkali,
H. Bouidida, A. Srifi, K.Taghzouti, Cherrah Y, et al. Anxiolytic plants in
Morocco: Ethnobotanical and ethno-pharmacological study. Phytothérapie
(2014).
7. Tutin TG,
Heywood VH, Burges NA, Valentine DH, Walters SM, et al.,Flora Europaea (1964)
Le complexe Mercurialis annua L. s.l. une étude biosystématique.
Annales des Sciences Naturelles, Botanique, Durand B (edtr) Cambridge
University Press, Cambridge, USA.
8. Krahenbuhl M,
Yuan YM, Kupfer P. Chromosome and breeding system evolution of the genus
Mercurialis (Euphorbiaceae): implications of ITS molecular phylogeny. Plant
Systematic and Evolution (2002) 234: 155-170.
9. Aquino R,
Behar I, DAgostino M, De Simone F, Schettino O, et al., Phytochemical
investigation on Mercurialis annua. Biochem. System. Ecol. (1987) 15:667-
669.
10. Novas ML,
Wolfman C, JH, De Robertis E. Proconvulsantand anxiogenic effects of n-butyl ß
carboline-3 carboxylate, an endogenous benzodiazepine binding inhibitor from
brain. Pharmacology Biochemistry and Behavior (1988) 30: 331- 336.
11. Perez RM,
Perez JA, Garcia LM, Sossa H. Neuropharmacological activity of Solanum
nigrum fruit. J Ethnopharmacol (1998) 62: 43-48.
12. Wolfman C,
Viola H, Paladini AC, Dajas D, Medina JH. Possible anxiolytic effects of
chrysin, a central benzodiazepine receptor ligand isolated from Passiflora
coeruiea.
Pharmacol Biochem Behav. (1994) 47:1- 4.
13. Kishore RN,
Anjaneyulu N, Naga Ganesh M, Sravya N. Evaluation of anxiolytic activity of
ethanolic extract of Foeniculum vulgare in mice model. Int J Pharmacy
Pharmaceutical. (2012) 4: 584.
14. Smith M,
Robinson L, Segal J. Anxiety medication.
15. Crawley J,
Goodwin FK. Preliminary report of a simple animal behavior model for the
anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav (1980) 13:
167-170.
16. Crawley JN.
Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev (1985) 9:
37-44.
17. Takeda H,
Tsuji M, Matsumiya T. Changes in head-dipping behavior in the hole-board test
reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol (1998)
350: 21-29.
18. Dunham NW,
Miya TS. A note on a simple apparatus for detecting neurological deficit in
rats and mice. J Am Pharm Assoc Am Pharm Assoc (1957) 46:
208-209.
19. Amos S, Adzu
B, Binda L, Wambebe C, Gamaniel K. Neuropharmacological effect of the aqueous
extract of Sphaeranthus senegalensis in mice. J Ethnopharmacol
(2001) 78: 33-37.
20. Perez RM,
Perez JA, Garcia LM, Sossa H. Neuropharmacological activity of Solanum
nigrum fruit. J Ethnopharmacol (1998) 62: 43-48. Keywords
Anxiety, Mercurialis annua, Rota rod test, Hole board test, Light–dark test, Morocco