Research Article :
The advent of the motorvehicle nearly one century ago has greatly altered modern life.
While modern industry and technology continue to develop, it is easy to enjoy
the benefits of these improvements rather than consider their consequences.
Motor vehicles, the most commonly used form of transportation, are highly
regarded for their convenience. However, motor vehicles produce whole body
vibration (WBV). Though motor vehicles have been greatly improved in recent
years, WBV does still exist and its potentially devastating aftermath must be
understood. In 2011, there was an estimated 5,338,000 police-reported motor
vehicle traffic crashes. According to a recent study by Charlie Klauer of the
Virginia Tech Transportation Institute Center for Vulnerable Road User Safety,
fatigue is the cause of 20 percent of all U.S. automobile crashes. Our study on
motor vehicle-induced-whole body vibration (MVWBV) challenges the previous
notion of driver fatigue [1,2]. These results suggest that “driver fatigue” I
actually brain impairment and dysfunction caused
by MV-WBV. To further this study, we hypothesize that MV-WBV can produce
accumulated brain injuries that compromise drivers cerebral function, such as
judgment and reactive capabilities, and can be the cause of motor vehicle
accidents. Long term MV-WBV is an important risk factor of cerebrovascular
diseases and stroke. On wide literature review, it is evident
that truck drivers have a higher incidence rate of hypertension,
cerebrovascular diseases and stroke [3-5]. Prevention of early neuronal injury
from MV-WBV can reduce late chronic brain diseases. In our previous study on
handarm vibration injury, the 4F-peptide was studied, and its preliminary
preventative effects from hand-arm vibration injury were described [6]. The
goal of this study is to understand the pathological damage to cerebral
capillaries and neurons from MVWBV and to validate the 4F-peptide
preventative effect from MV-WBV injury. A study of simulated animal vibration
is an effective way to understand this pathologicalprocess and damage as rats are similar to humans in anatomy and
physiological and biological features, which has been demonstrated quite
effectively in the past [7-9]. For the care and use of laboratory animals,
all protocols in this study conformed to the National Institutes of Health
(NIH) guidelines and received approval from the Biomedical Resource Center
(BRC) and the Institutional Animal Care and Use Committee (IACUC) at the
Medical College of Wisconsin (AUA- 2363). After the animals arrived, they were
allowed to acclimate for 7 days prior to exposure. Animals were housed in a
central animal care facility with 12-h light cycles and given food and water
adlibitum. Animal groups 56 Sprague-Dawley male rats (weight
250-300g) were divided into seven groups (N=8): (1) 8-week normal control: had
no treatment; (2) 8-week vibration group (exposed to whole body vibration at 30
Hz and 0.5g acceleration for 4 hours/ day, 5 days/week for 8 weeks); (3) 8-week
vibration group with preconditioning 4F peptide; (4) 8-week vibration group
with post conditioning 4F peptide; (5) 8-week vibration group with pre and post
conditioning 4F peptide; (6) 12-week normal control group; and (7) 12-week
vibration group (exposed to the same vibration, 5 days/week for 12 weeks). At
the end point, all rats were evaluated by brain histo-pathological studies. Vibration set-up The rats in all control and vibration
groups were placed individually in poly vinyl chloride (PVC) tubes. Rats
voluntarily entered the tubes. The rats did not exhibit stress at all as seen
in our previous rat experiments. The tubes were taped to a vibrating platform
and the tails were taped alongside the tube on the platform (Figure 1).
Vibrations were performed without any sedative or anesthesia. The electromagneticvibration motor (Bruel and Kjaer (B&K), type 4809, Denmark)
was driven by a sine wave signal from a function generator (Simpson 420, Elgin,
IL). The acceleration was set with a power amplifier (B&K type 2706).
Frequency and acceleration were calibrated prior to beginning the study using
an HP 1201 B oscilloscope and a B&K 4384 accelerometer connected to a
B&K Integrating Vibration Meter, type 2513, with linear vertical
oscillations of 30 Hz and 0.5g acceleration (4.9 m/s2 r.m.s. acceleration).
These vibration parameters were selected after wide literature review and
consultations with US and international human-WBV associations; therefore the
vibration parameters chosen simulated the most common motor vehicle driving
situations [10-17]. The reliability of this animal model for vibration studies
has been identified and compared to other vibration animal models demonstrating
that this model is psychologically stressfree by our systematic and continued
studies described in our published paper [10]. Conditioning rats with 4F (human
apolipoprotein A-I molecule mimetic)
The
4F (Amino acid sequence: Ac-DWFKAFYDKVAEKFKEAFNH2) was synthesized by the Blood
Center of Wisconsin. The peptide was reconstituted in normal sterile saline.
The preconditioning group received subcutaneous injections of 4F (3mg/kg) every
day approximately 30 minutes prior to the daily exposure. Rats were weighed
daily to adjust the dosage of 4F as required. The rationale for the
administering paradigm of 4F to the rats was as follows: oral dosing in the
food or water would not provide enough accuracy; therefore, we administered 4F
as a subcutaneous injection to verify the exact amount given to each rat every
time. The dosing concentration of 3mg/kg has been shown to be quite effective
in reducing the effects of vibration injury as found by our previous study. It
is similar to the concentration used in human studies and is at the lower end
of other published in vivo studies 4F [6]. The published 4F half-life times
vary from 6 to 12 hours. Our hypothesis for the timing of injections is that
preconditioning might be most important because the initial WBV effect is
vasospasm and 4F is a strong vasodilatorand neuralprotector, which prevents myelin damage as shown in our previous
published work on vibration [6]. Having a high circulating concentration of 4F
at the onset of vibration might prove to be the best prevention approach. In
addition, a recently completed clinical trial titled: “4-EVER: a Trial
Investigating the Safety of 4F Endovascular Treatment of Infra-Inguinal
Arterial Stenotic Disease” (ClinicalTrials.gov Identifier:
NCT01413139) has shown positive results. Tissue processing for Histo-pathological study Light microscopic (LM)
and transmission electron microscopic (TEM) studies on the brain cortices were
performed after vibration had completed. At the end time point, the rats were
anesthetized using isoflurane gas inhalation. The rat skull was carefully
opened using a microsurgical saw under a surgical microscope. On the right
side, the right brain cortex (1 mm thickness) was carefully harvested using a
#11 blade without any traumatic intervention. Specimens were immediately immersed into a 2.5% glutaraldehyde
solution in phosphate-buffered saline. After fixation, they were processed
routinely and plastically embedded. Semi-thin 0.5μm transverse sections were
cut and stained with toluidine blue for the LM study. Ultra-thin (50-70 nm) sections
of the brain cortex, including capillaries, stained with salts of uranyl
acetate and lead citrate were prepared for the TEM study. The left cortex
tissue was harvested in the same way for H & E (hematoxylin and eosin)
stain. The brain membrane was kept intact to avoid artifact neuronal injury and
immediately immersed in the 10% formalin in 0.1 M phosphate-buffered saline at
pH 7.4. The tissues were then processed for routine paraffin embedding. Blocks
were stored at 4°C until H & E staining for cortex neuron injury analysis. Measurement of capillary dilation degree In the semi-thin
sections, the capillary dilation degree wascalculated as a ratio. The inner
endothelial circumference (EC)and the outer pericyte circumference (OC) were
measured usingMeta Vue Software (Molecular Devices, Sunnyvale, Ca). Each
circumference was manually traced in Meta Vue, and the lengthof each parameter
was then calculated by the program. Thecapillary dilation ratio was calculated
by dividing EC/OC for eachcapillary. A lumen size change is indicated by the
squeezing of theendothelium with an unchanged OC. This is a proven
accuratemeasurement [18,19]. A lower mean EC/OC Ratio indicates
constricted lumen, and the capillary spasm in the acute WBV stagemay become
structural constriction in the chronic WBV stage. Neuronal pathological analysis There were two types of
histo-pathologically prepared sections for study: 1) semi-thin sections were
observed for cerebral microvascular changes and 2) H & E stained sections were observed and
analyzed for neuron changes. Results General behavior and welfare of all
animals were monitored daily. No animal showed abnormal behaviors of stress
such as shaking, clenching, biting, postural arching, or clawing. Health
inspections included checking for secretions of eyes/nose, porphyrin discharge
(chromodacryorrhea), and hematuria. No animal presented any of these ill
appearances nor exhibited symptoms of persistent diarrhea, weight loss, light
avoidance, lethargy, head waving (inner ear infection), or congested breathing.
The vital signs, such as heart rate, respiratory rate, and oxygen saturation of
all rats remained stable. Their eating and drinking habits did not change. The
growth rate of all rats was within the normal range. This intense observation
has shown that this animal model is stress-free, which was further confirmed by
our previously published work [6,10,19]. In our subsequent studies, only the
WBV factor was tested. Pathological features in different vibration terms In both the 8- and
12-week controls (Figure 2A and 2C), the capillaries displayed well-opened
oval-shaped lumens with clearly intact basement membranes (BM) of the
endothelial cells and cell membranes of the pericytes. The endothelium cell
layer of the capillary also maintained a uniform thickness. All of these pathological
features suggest no brain impairment or dysfunction. With WBV, noticeable
damage was sustained by cerebral capillaries. In the 8-week vibration group the
brain capillaries had significant characteristic changes in all the rats
compared to a normal control: the endothelium layer of the capillary walls was not
of uniform thickness and was partially damaged and broken, the lumens were
severely constricted, and edema was present around the capillaries and all
neuropils. The capillary walls were also thicker (Figure 2B and 2D). All these
features indicated that capillary sclerosis had occurred, and the blood
perfusion of the whole brain was dramatically compromised. Each of
these effects was more pronounced in the 12-week vibration group (Figure2D) as
compared to the 8-week vibration group (Figure 2B). The nueropil backgrounds of
both the 8- and 12-week vibration groups are less intense than in the normal
controls and vacuoles can be seen; all of these changes are more prominent in
the 12-week vibration group, as many vacuoles can be seen in the neuropils (Figure
2D). These characteristic changes indicate extensive chronic edema occurred in
the 12-week vibration group. The capillary dilation ratio of the 8-week
vibration group was 0.595, and this was smaller than the 8-week control group
(0.690); this comparison was statistically significant (p=0.003). A similar comparison
pattern was also visible in the vasodilation ratios of the 12-week control vs.
vibration groups (0.721 vs. 0.572, respectively) and this was also
statistically significant (p<0.0001).The capillary dilation ratio of the
12-week vibration group was 0.572, which was less than the capillary dilation
ratio of the 8-week vibration group (0.595), suggesting that longer periods of WBV
cause more damage to brain capillaries and lead to increased cerebral
impairment by thickening capillary walls and shrinking lumen size to a greater
extent. Compared to the 8 week vibration group (Figure 2B), the 12-week
vibration capillary (Figure 2D) has a more uneven endothelium layer of the
capillary wall. Therefore, more damage occurs to cerebral capillaries and brain
impairment occurs to a greater extent with longer periods of WBV. The rats that
were conditioned with the 4F peptide before and/ or after vibration had a larger
degree of capillary dilation than the 8-week control and 8-week vibration
groups. The endothelium layer of these capillaries was also of a uniform thickness and was not damaged or broken. Compared to
the 8-week control capillary. Figure 3: Cross-section of cerebral capillaries. A: 8-week vibration with
pre-conditioning 4F peptide. B: 8-week vibration with postconditioning 4F
peptide. In both pictures, the solid arrow points to red blood cells. The
dashed arrow is pointing to the lumen, which is not constricted in these
capillaries. The solid arrowhead is pointing to the capillary wall; in these
capillaries, the endothelial layer is of a uniform thickness around the entire
capillary. Bar = 50 µm.
Table 1: Capillary dilation ratio (EC/OM).
Capillaries, as vascular end units, are directly relevant to tissue perfusion,
providing oxygen to meet the bodys needs. This study focused on the capillary
changes that result from WBV. From our previous study, we found that in shorter
periods of vibration (one to two weeks), there is no obvious capillary damage.
However, significant damage does occur to capillaries when vibration periods
equal or exceed four weeks [20]. As seen in figure 5A (from our previous
study), the basement membrane of the 4-week control group is clearly defined.
However, in the 4-week vibration group (figure 5B) the basement membrane has
nearly disappeared, the cell membranes of the pericyte cells have become coarse
and thick, and the entire capillary wall is now thicker and more fibrotic.
Therefore, noticeable cerebral damage is sustained after the 4-week vibration
period. The insidious nature of the WBV injury coupled with its discretely
identifiable sequelae should be seriously regarded. Acute WBV causes cerebral
vascular spasm, leading to constriction and ultimately to decreased cerebral
blood flow. Neurons begin to sustain impairment. Drivers usually feel fatigued
and drowsy during long hours of driving. We believe this brain dysfunction
(impairment) is from WBV, which decreases judgment and reactive capability
suggesting that it is an important contribution to “driver fatigue.” The exact
biological mechanism of WBVinduced vascular and neuronal damage is still
unknown. Locally, vibration force both stimulates sympathetic nerve fibers to
release norepinephrine and increases the smooth muscle sensitivity to
norepinephrine, a twofold amplification of norepinephrine efficacy [21]. The
smooth muscle spasm may also be caused by direct stimulation from the vibration
shearing force: endothelial cells are physically injured during
vasoconstriction by tight pinching between the folds of the internal elastic
membrane, which causes bulging into the lumen [22]. The damage to neurons and
peripheral nerves is from direct shearing force and ischemia. Relative to the
average rat lifespan of 3 years, the average human lifespan in the US is 78
years, which is about 26 times that of the rat lifespan. 8- and 12-week vibration
periods in the rats are equivalent to approximately 4.5 and 6 years of
vibration to the human respectively. Four and a half years for most
occupational drivers is not a very long period, rendering a significant
comparable timeline in our 8-week rat models. Furthermore, our pathological
study has shown this injury is a gradually cumulative process, as our measured
changes at 12 weeks were significantly greater than 8 weeks. These
morphological lesions such as thickening and destruction of capillary walls may
permanent brain lesions and other secondary diseases, such as chronic
hypertension, cerebrovascular diseases, and stroke in the human model. This may
suggest that damaging processes such as WBV may play a role in other cerebral
diseases and pathologic processes much more extreme than mere drivers fatigue,
such as dementia, Alzheimers disease, or even Parkinsons disease. Early
preventative approaches, such as the use of protective peptide 4F, could be
utilized and potentially stymie these destructive processes. Such future
studies, perhaps in human models, would be greatly beneficial to understanding
the pathophysiology of these mysterious cerebral disorders. U.S.
Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine
& Advanced Technology Research Center (TATRC), Fort Detrick, MD, [Grant
number W81XWH111069]. No competing financial
interests exist for all authors. Cumulative brain injury; Brain dysfunction; Whole-Body Vibration
Insidious Cerebral Capillary Trauma from Motor Vehicle-Induced Vibration
Abstract
Cumulative cerebral injury from
motor vehicle-induced whole body vibration has not been demonstrated thus far.
Our lab has demonstrated isolated cerebral injury from whole body vibration and
we believe that repetitive vibration can cause cumulative insults, impairing
cerebral function. A simulated motor vehicle-induced whole body vibration study
was conducted with fifty-six Sprague-Dawley rats divided into seven groups
(N=8): (1) 8-week control group; (2) 8-week vibration group (exposed to whole body
vibration at 30 Hz and 0.5g acceleration for 4 hours/day, 5 days/week for 8
weeks); (3) 8-week vibration group with preconditioning human apolipoprotein
A-I molecule mimetic (4F); (4) 8-week vibration group with post conditioning 4F
peptide; (5) 8-week vibration group with pre and post conditioning 4F peptide;
(6) 12-week control group; and (7) 12-week vibration group (exposed to the same
vibration, 5 days/week for 12 weeks). At the end point, all rats were evaluated
by brain histo-pathological studies.
The pathology
demonstrated capillary constriction with surrounding edema as well as
thickened, uneven and damaged capillary walls. Capillary constriction reduces the
oxygen supply to cerebral neurons, leading to neuronal damage. In the 12-week vibration
group, each effect was more pronounced as compared to the 8-week vibration group.
There was no prominent cerebral capillary damage in the 4F-peptide conditioning
groups. This study showed cumulative cerebral injury from motor vehicle-induced
whole body vibration and demonstrated the preventative effect of 4F-peptide
conditioning.
Full-Text
Introduction
Materials and Methods
Ethics statement
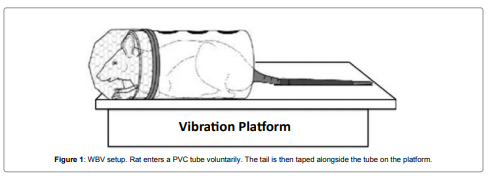
Figure 1: WBV setup. Rat enters a PVC tube voluntarily. The tail is then
taped alongside the tube on the platform.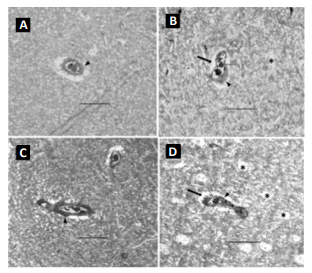
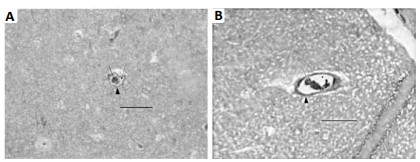
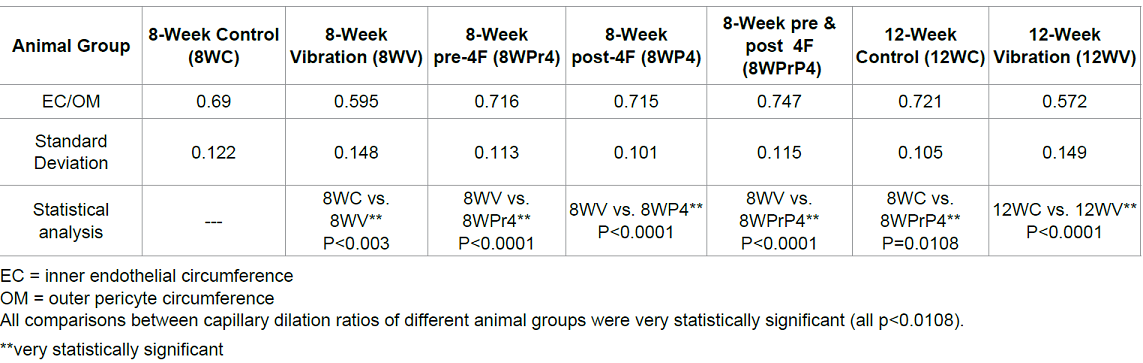
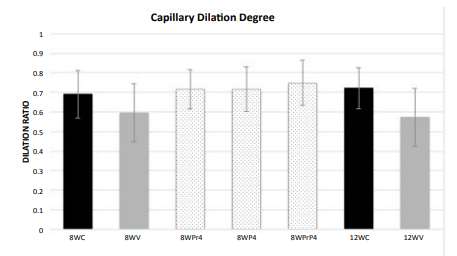
Figure 4: Capillary Dilation Ratio
of each animal group. 8WC: 8-week control; 8WV: 8-week vibration; 8WPr4: 8-week
pre-conditioning 4F peptide; 8WP4: 8-week post-conditioning 4F peptide; 8WPrP4:
8-week pre- and post-conditioning 4F peptide; 12WC: 12-week control; 12WV:
12-week vibration.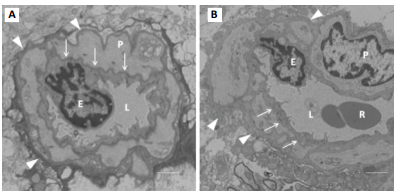
Figure 5: TEM section of cerebral
capillary. P = pericyte cell, E = endothelial cell, R = red cell, L= lumen. A
was from 4-week control group: Arrows indicate basement membrane (BM) that was
a clear intense black line; B was from 4-week vibration group: Its BM was
invisible or disappeared. The arrowheads indicate cell membrane of pericyte
cells that was also clear and intense black structure in A, while in B this
became a coarse, thicker gray structure. The whole capillary wall apparently
presented as thicker and more fibrotic in B than in A. Bar = 1 µm.
Acknowledgment
Author Disclosure Statement
References
Keywords