Research Article :
Background: Tuberculosis, a disease of significant public
health importance remains a leading cause of childhood morbidity and mortality
globally. Under-reporting of new cases is a major setback in the correct
estimate of the global burden of pediatric TB. Data on pediatric TB from
TB-endemic countries being limited. It is recommended that continuous research
be conducted to ascertain and better understand the magnitude of the problem
and to provide reliable, timely and cost-efficient information for action. Objective: This study was undertaken to determine the
bacteriological prevalence of tuberculosis and TB-HIV co-infection among
children seen in health facilities in Nasarawa state, North-Central Nigeria. Subjects and Method: The study subjects consisted of one hundred and
fifty (150) children aged 18 months to 15 years who were selected using
multi-stage sampling technique. Data, was obtained from their care-givers using
interviewer administered questionnaires. The study subjects had their sputum or
gastric aspirates samples collected for acid-fast bacilli microscopy and
culture. Blood samples were taken for HIV screening. Data was analyzed using
SPSS statistical software version 17.0. Results: The ages of the subjects averaged 9.12±4.66 years
and majority of them were females with male to female ratio of 0.92:1. The
prevalence of tuberculosis found among them by microscopy and culture are 16.7%
and 30.0% respectively while the prevalence of definite TB case was 32% which
is 5.5 times higher than the reported national average. The prevalence of 10.0%
for TB-HIV co-infection was also found among the study subjects. Conclusion:
There is a high burden of pediatric TB in Nasarawa State (higher than average
national prevalence). This study can be extended to six Geopolitical regions of
Nigeria, to find out the true situation nationwide. Tuberculosis is the first health condition that
was declared by World Health Organization (WHO) as a “Global Emergency”. WHO
gave a global estimate of TB incidence in the year 2008 to be 9 million cases
annually, and of this, about 1 million (11%) occur in children (under 15 years
of age) [1-3]. Of these childhood cases, 75% occur annually in 22 high-burden
countries that together account for 80% of the worlds estimated incident cases;
Nigeria is listed among these countries. Under-reporting was the major setback
in the WHO correct estimate of global burden of pediatric TB cases because of
the diagnostic challenges and poor record keeping among high disease burden
countries [1]. The diagnostic challenges are worsened by the undue reliance on
sputum smear microscopy for diagnosis whose yield is poor in children
(<10-15%) due to the paucibacillary nature of their TB, and in most cases
are unable to produce sputum. Thus, this inadvertently keeps out more than 95%
of TB cases among children who are younger than 12 years from being diagnosed
[2-4]. Approximately 2 million persons globally die
each year from active TB despite the existence of effective treatments for both
latent infection and active disease, and more than half of all deaths occur in
Asia. Every 15 seconds, one of every three persons to die from TB is a child
[2,3]. Second to Asia, Africa appears next on the list of continents with high
prevalence of TB. The number of incident cases in the African Region is still
on the increase with estimate of 2.6 million (29% of global burden) in 2013
compared with 2.3 million (25.5% of global burden) in 2008 [2-5]. According to the 2014 WHO global tuberculosis
report, Nigeria was ranked 3rd
among the 22 high TB burden countries globally and the 1st in Africa with
incidence of 340,000-880,000 [5].This
estimate is however approximately two times higher than the previous estimates
of 2011 and this underscores Nigerias high TB burden. Although over-diagnosis
does occur, under-diagnosis is more frequent especially among children due to
diagnostic challenges. This problem of under-diagnosis in children is clearly
seen by the low national reported pediatric caseload of 1.4% in 2011 and 5.8%
in 2013 [5,6].The total number of
cases and deaths are still rising due to population growth,therefore there is need to ascertain the
present prevalence in our environment [7].
This study was a health facility based carried
out in Nasarawa State North-Central Nigeria. The climate is tropical and the
vegetation is Guinea Savannah which is favorable for rearing of livestock with
seven Grazing Reserves [8]. The central towns in the state
have grown in the recent time into major urban centers with a high population
influx as a result of ethno-religious crises and insurgents terrorist attacks
in North-Eastern Nigeria. These towns are now major truck stops for long
distance drivers and a beehive of social and commercial activities in the midst
of low literacy and high fertility rate [9-12]. The study design was a facility-based
cross-sectional descriptive study that was carried out over a period of six
months; February 2012 to July 2012. The sample size was calculated to be 150
children based on the 8.9% prevalence of TB in children taken from the reported
percentage of smear positive microscopy in the bacteriology study of childhood
TB in Ibadan, Nigeria [13]. A multistage sampling technique was used to
select the study subjects. Stage one was the selection of 3 LGAs, one from each
of the senatorial districts by simple random sampling (SRS) technique using a
table of random numbers. The selected local governments were Lafia, Keffi and Akwanga. Stage two was the selection of six health
facilities, two each from the 3 LGAs by SRS technique by balloting. Stage three
involved the selection of the study subjects using systematic sampling
technique in which the initial subject who met the eligibility criteria was
selected using SRS by balloting, after which the sampling interval of 2 or 3
(as calculated for each LGA) was used to select eligible subjects until the
predetermined sample size for the health facility was reached. A record was
kept of the names, gender and hospital number of selected subjects so as to
avoid reselection of subjects during the next clinic or hospital visits. The inclusion criteria were
children age 18 months to 15 years old seen in the six selected health
facilities in three LGAs in Nasarawa state; written informed consent,
including readiness to comply with the processes of sample collection. These included subjects who were receiving in-patient and
ambulatory services. Children who are on Anti-tuberculosis chemotherapy
were excluded from the study because it may affect the bacteriology giving
false negative results. Ethical clearance for the study was obtained
from the Human Research and Ethical Committee of the Nasarawa state Ministry of
Health. A written informed consent was sought from
parents/caregivers in addition to child accent. The nature of the study, aims
and objectives were explained in detail to the parents/caregivers/subjects by
the researcher and where necessary interpreter is used. Where they decided to opt
out at any stage of the study they were free to do so and they were not
deprived of any consultation or treatment. Counseling of subjects and caregivers on
HIV/AIDS was done in two phases which are pre- and post-test. Where the result
is positive, information like confirming the diagnosis, treatment options, care
of the child, screening of parents/siblings for HIV, issues concerning
disclosure and shared confidentiality were discussed with caregiver /subject to
help them decide on a realistic course of action that is suitable for the
family. A designed questionnaire was administered to
get information on personal data, medical, and family history from each subject
in each of the selected health facility. Specimens of either sputum for subjects ≥5
years or gastric wash-out for subjects <5 were used for Bacteriological
study which includes Acid Fast Bacilli (AFB) microscopy using Ziehl–Neelsen stain and mycobacterium
culture on Egg-basedmedia (Löwenstein
–Jensen) in the reference laboratory in Jos University Teaching Hospital
(JUTH). Resultswere
recorded in the questionnaire form of each subject. For the
purpose of international standardization in line with WHO recommendation, a
definite tuberculosis case
definition was adopted for reporting TB cases in this study. This is simply
defined as any subject with either culture positive or AFB microscopy positive
in two of his/her samples or has the combination of positive results in both
culture and AFB microscopy. In other words, a case of TB is reported in this study as AFB microscopy positive (in
two smears)+culture negative, or culture positive+AFB microscopy negative, or AFB
microscopy positive (in two smears)+culture positive [14]. All
subjects data in the questionnaire form were entered into the computer using Microsoft
excel and analysed using the SPSS statistical software version
17.0. Categorical variables were cross tabulated using frequencies and
percentages, whereas quantitative variables were summarized using mean,
standard deviations, median or range as appropriate. The prevalence of TB and
TB/HIV co-infection were expressed as proportion. Student t-test was used for
comparison of means of variables. The chi square test was used for testing
significance of association between categorical variables on contingency
tables. All tests of significance were two-tailed. P-value <0.05 was taken
to indicate statistically significant difference. A total of 150 subjects were enrolled into the
study with mean age of 9.12±4.66 years and median age of 10.0 years. Of the 150
subjects studied 72(48%) were males while 78(52%) were females with male to
female ratio of 0.92:1. Sputum and gastric aspirates samples were
collected from 110 and 40 of the study subjects respectively. Out of the 110
sputum samples 41 (37.8%) were found to have definite TB, whereas from 40
gastric aspirates samples 7 (17.5%) of them were diagnosed of definite TB and
this is statistically significant (p=0.022). The prevalence of tuberculosis in the study
population by microscopy and culture were 25/150 (16.7%) and 45/150 (30.0%)
respectively while the prevalence of definite TB case is 48/150 (32%). The prevalence of TB/HIV co-infection is
15/150 (10.0%). AkwangaLGA
has the highest TB prevalence among its population 15/39 (38.5%) followed by
Lafia and Keffi LGAs with prevalence of 22/70(31.4%) and 11/41(26.8)
respectively, but the difference is not statistically significant (p =0.532) as
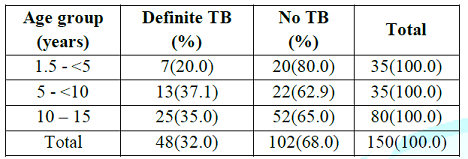
shown in Table 1. Table 1: Prevalence of definite TB by age groups of study subjects. Within the age groups, children between the
ages 5 - <10 years have the highest prevalence of definite TB (37.1%)
compared to under fives (20.0%) and the adolescents (35.0%) but this difference
is not statistically significant (p= 0.215). For the
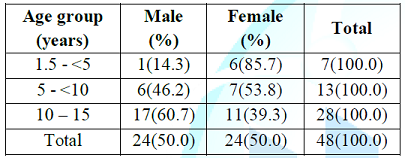
age and sex distribution of definite TB Cases, equal number of males (24) and
females (24) were diagnosed. Amongst the males with definite TB, the
adolescents have the highest prevalence (60.7%) followed by the age group 5-<10
years (46.2%) and under fives (14.3%). For the females under-fives have the
highest prevalence (85.7%) followed by the age group 5 - <10 years (53.8%)
and adolescents (39.3%). There was no statistical significance (p=0.085) in the
differences of the age and sex distribution as shown in Table 2.
Table 2: Age and sex distribution of definite TB cases (n=48). The
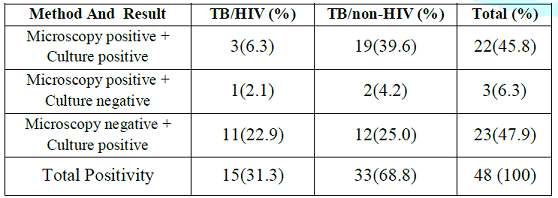
correlation of results of AFB microscopy and culture for TB/HIV and TB/non-HIV
among definite TB cases (n=48) is shown in Table 3. More Acid-fast bacilli were detected by
culture 23/48 (47.9%) than microscopy 3/48 (6.3%). AFB culture had a higher
yield amongst TB/HIV co-infected (22.9%) and TB/non–HIV infected (25.0%)
compared to microscopy which had a yield of 2.1% and 4.2% respectively. These
differences were statistically significant (p =0.047). The
validity testing of AFB microscopy (Ziehl Neelson) and culture (Löwenstein–Jensen) found in this study
shows that culture has higher sensitivity (95.7%) and NPV (98.1%) than AFB
which has sensitivity of 52.0 % and NPV of 81.6%. However, both have specificity
and PPV of 100%.The differences were statistically significant (p = 0.0001). The thirty two percent prevalence of definite
TB found among children in Nasarawa state, North central Nigeria is higher than
the ones reported previously in other regions of the country which were 8.9%
(Ibadan, South-West), 22.1% (Benin, South-South) and 1.1% (Sokoto, North-West)
[13,15,16]. The use of culture method of diagnosis in this study is a possible
explanation for the higher prevalence obtained compared to the previous studies
cited in that TB culture is known to increase the yield of acid-fast bacilli
even when missed by microscopy [17]. Although Ibadan, as previously cited,
included culture method of diagnosis; it was characterized by high culture
contamination rate which resulted in low yield [14]. The TB prevalence in this study when compared
with the reported 2013 national prevalence rate of 5.8% among children is 5.5
times higher [6,18]. This could be due to the inclusion of some DOTs centres in
the study which have the capacity to diagnose pediatric TB, and are known to
have contributed about 85% of reported TB cases seen in children in the state [19].
The strongest reasons however may lie in the fact that the risk factors for TB
exist in high proportion in Nasarawa state. These factors includes the very
high HIV infection sero-prevalence of 10.0% among pregnant women attending
antenatal clinicin Nasarawa state making it second highest in the nation
according to the 2010 national technical report [20]. Other prevailing risk
factors are high level of poverty, unhealthy living conditions, low BCG
immunization coverage, low maternal educational level; and the recent influx
and increasing migrant settlements in Nasarawa state from conflicts areas of
the North [10-12]. There has also been the report of increased consumption
ofunpasteurized milkand increased close contact of general populace with
confirmed TB infected herds [8]. Also, the fact of national prevalence rate
being lower when compared to the rate found in this study may have emanated
from the undue reliance on the compilations of data solely from DOTs programme
registry of different states which may not give a true reflection of the pediatric
TB burden in the country [1,6,21,22]. Moreso, some of these DOTs centres are
known to report low TB rates for children as a result of diagnosis challenges
coupled with the paucibacillary nature of pediatric TB, inability of children
to produce sputum for bacteriology, difficulties often encountered in obtaining
other samples such as gastric washouts, lymph nodes biopsy etc and the
inadequate number of trained personnel to make diagnosis of TB [3,4,19,23]. Similarly, the recommendation of national
tuberculosis control programme (NTCP) for the use of CXR and TST (mantoux test)
as basic tools for diagnosis especially among children with smear-negative TB
disease, though widely used is not without its limitations [1,24]. These
limitations are also possible strong factors responsible for the under
diagnosis and under reporting of pediatric TB in the state and the nation at
large, thus reducing the impact of DOTs services among children. The
limitations can be traced to; firstly, the unavailability of tools in endemic
rural areas with limited resources. Secondly, CXR interpretations are marked by
inconsistencies (both inter and intra observer variability), and its
reliability depends on expertise of the interpreter; who are mainly
concentrated in tertiary health facilities in urban settings. For TST (mantoux
test), the tool is not specific for M. tuberculosis infection, and not
sensitive in immune-compromised children. Finally, NTCP recommendation for the
use of clinical score charts has challenges nationwide due to poor knowledge on
its use and application among primary health workers, its poor validity and
poor performance particularly in children suspected of pulmonary TB (the most
common form) and in children who are also HIV infected [24,25]. These factors,
coupled with poor attitude toward record keeping, contact investigation and
routine surveillance have contributed remarkably to the problem of under-reporting
of pediatric TB in Nigeria as reflected in the very low national prevalence
rate despite the nations ranking as the fifth among the twenty two high TB
burdened countries globally and the second in Africa [5,6]. Therefore the
exceptionally high prevalence of TB found in the present study supports the
assertion that national average notification figures often may not reveal the
disparity in case rates between other parts of the country [26]. The growing association between TB and HIV is globally
recognized [17]. The prevalence of TB-HIV co-infection found in this study is
10.0%. This can be explained by the fact that the state is ranked second
highest in terms of HIV prevalence in the country and therefore high rates of
TB-HIV co-infection are not unexpected as TB is one of the common opportunistic
diseases associated with HIV infection [17,27]. Comparing the prevalence of
TB-HIV co-infection found in this study to that reported in Cape Town, South
Africa by Schaaf et al (2007) [28], shows that the prevalence is approximately
twice lower than what was reported from Cape Town where TB-HIV co-infection was
found to be 22.3% amongst culture confirmed children. The probable reasons for
this discrepancy could be higher prevalence of HIV infection in the general
populace of Cape Town as demonstrated by HIV sero-positivity of 15.7% amongst
women attending public antenatal care facilities (Cape Town) compared to 10.0%
in Nasarawa state, and also from declining rates of HIV in Nigeria from
effective intervention programs [6,28]. Furthermore the use of more advanced
culture medium (Middlebrook 7H9 broth based) in the study from Cape Town could
account for higher yield for AFBs and therefore higher prevalence of TB/HIV
co-infection. It was also discovered that, the yield of AFB
by sputum production is twice higher than gastric aspirate in this study. This
finding is consistent with the report of the study on culture-confirmed
childhood TB in Cape Town, South Africa by Schaarf et al. in 2007 [28]. The
reason for this could be that sputum samples of TB infected children are
heavily laden with AFBs in that it proceeds directly from the lungs without
undergoing degradation along it route and few as 10 bacteria/millilitre can be
detected by culture [29]. Also TB infected children with ability to produce
sputum are usually older children who in most instances have adult form of TB
(open TB) [18]. As for gastric aspirate, AFBs are degraded in the acidic
environment of the stomach during the overnight fast thereby reducing the
quantity of AFBs in the sample [30]. Also children in whom early morning
gastric washings are performed usually have paucibacillary form of TB and
couple with the fact of diluting samples with 10-20 ml of normal saline during
procedure reduces the concentration of bacteria/milliliter thereby affecting
the overall yield of AFBs from gastric aspirates [30,31]. Consequently, correlation of results of AFB
microscopy and culture for TB/HIV co-infection and TB/non-HIV infected subjects
show that more AFB are detected by culture alone than microscopy with
approximately fourty two percent of the AFB missed by microscopy being detected
by culture. These data corresponds to observations recorded in a report by
Onubugu et al. in 2010 [17]. Added to this fact, the high sensitivity of
culture in contrast to microscopy found
in this study demonstrate the superiority of culture method of diagnosis over
microscopy and therefore suggest that culture of Mycobacterium tuberculosis
remains the gold standard in pediatric cases. Summarily, the health implication of the high
TB prevalence found in this present study shows that extra efforts and
commitment are needed in our national tuberculosis control programme geared
towards intensifying intervention and TB control measures in states with high
prevalence based on data generated from periodic research, otherwise high case
rates are likely to continue. The fact that more AFBs are detected by culture
than microscopy is an indication that there is an urgent need for the country
to increase capacity for culture facilities in TB laboratories and provision of
long awaited GeneXpert MTB/RIF machine with appropriate cartridges for childrens
different samples such as Gastric washouts, Cerebrospinal fluids, pleural
fluids etc. This will avert the consequences of undue reliance on microscopy
that include delayed or misdiagnosed cases, contributing to delay in treatment
and increased morbidity and mortality rates among children. 1.
Nelson
LJ and Wells CD. Global epidemiology of childhood tuberculosis (2003) Int J
Tuberc Lung Dis 8: 636-647. https://dx.doi.org/10.1186%2Fs41479-016-0018-6 2.
WHO.
Global tuberculosis control; Epidemiology, strategy, financing: WHO report
(2009) Geneva, Switzerland: WHO Health Organization. 3.
Stop
TB partnership. 2009 updates tuberculosis fact sheets (2009) World Health
Organization. 4.
WHO.
Global Tuberculosis control: Surveillance, planning, financing. WHO Report
(2004) Geneva, Switzerland: World Health Organization. 5.
WHO.
Global tuberculosis control report 2013 (2013) Geneva: World Health
Organization. 6.
WHO.
Global tuberculosis control report 2011 (2011) Geneva: World Health
Organization. 7.
Full
WHO Data. Estimated Epidemiological burden of TB 1990-2008 (2008) WHO. 8.
Yohanna
CA, Ijabone IF and Cadmus SIB. Prevalence of bovine tuberculosis using single
comparative intradermal tuberculin test (SCITT) in Fulani herds in Nasarawa
state, north central Nigeria (2008) Sokoto J Veterinary Sci 7: 46-48. 9.
Wikipedia,
the free encyclopedia (2010) Nasarawa State 10. National Bureau
of Statistics (2010) Nigeria. 11. Laah JG and Ayiwulu
E. Socio-Demographic characteristics of patients diagnosed with HIV/AIDS in
Nasarawa Eggon (2010) Asian J Med. Sci 2: 114-120. 12. National
Population Commission and ICF Macro (2009) Nigeria Demographic and Health
Survey 2008 Fact Sheet: North Central. Abuja, Nigeria. 13. Kehinde AO,
Oladokun RE, Ige OM, Bakare RA and Osinusi K. Bacteriology of Childhood
Tuberculosis in Ibadan, Nigeria: A five-year review (2008) Tropical Med Health
36: 127-130. http://dx.doi.org/10.2149/tmh.2008-11 14. WHO. Guidelines
for HIV surveillance among tuberculosis patients (2004). 2nd ed. WHO
document WHO/HTM/TB/2004.339. 15. Ibadin MO and Oviawe
O. Trend of childhood tuberculosis in Benin City, Nigeria (2001) Ann Trop Pediatric
21: 141-145. https://doi.org/10.1080/02724930120058205 16. Jiya NM, Bolajoko
TA and Airede KI. Pattern of childhood tuberculosis in Sokoto, North western Nigeria
(2008) Sahel Medical J 11: 110-113. http://dx.doi.org/10.4314/smj2.v11i4.12982 17. Onubogu CC,
Kunle-Ope CN, Onyejepu N, Nwokoye NN, Raheem TY, et al. Prevalence of
tuberculosis and human immunodeficiency virus (TB/HIV) co-infections amongst
patients with brochopulmonary disorders in Lagos (2010) African J Microbiol Res
4: 1904-1908. 18. Carlos MP and Marais
BJ. Current concepts of tuberculosis in children (2012) N Engl J Med 367:
348-361 19. Nasarawa State
Ministry of Health. The Data/Statistics and Indicators for Tuberculosis: Five
years review (2008-2012) (2012) Nasarawa Tuberculosis and Leprosy control
programme, Lafia. 20. Federal Ministry
of Health. Technical Report on the 2008 National HIV/Syphilis Sero-prevalence
Sentinel Survey Among Pregnant Women Attending Antenatal Clinics in
Nigeria. Department of Public Health
National AIDS/STI Control Programme (2010) Abuja: Nigeria. 21. Dolin P,
Raviglions MC and Kochi A. Global tuberculosis incidence and mortality during
1990-2000 (1994) Bull World Health Organ 72: 213-220. 22.
Ani
AE. Overview of diagnostic methods and distribution of mycobacterium tuberculosis in Nigeria (2009) Transactions of the
Royal Society of Tropical Medicine and hygiene.
23. Marais BJ, Graham
SM, Cotton MF and Beyers N. Diagnostic and Management Challenges for Childhood
Tubercolosis in the Era of HIV (2007) J infectious diseases 196: 576-585. https://doi.org/10.1086/518659 24. Marais BJ and Madhukar
P. Recent advances in the diagnosis of childhood tuberculosis (2007) Arch Dis
Child 92: 446-452. https://dx.doi.org/10.1136%2Fadc.2006.104976 25.
Kristina
F and Lisa S. Tuberculosis in children (2005) Clin Chest Med 26: 295-312. http://dx.doi.org/10.1016/j.ccm.2005.02.010 26. Guwatudde D,
Zalwango S, Kamya MR, Debanne SM, Diaz MI, et al. Burden of tuberculosis in
Kampala, Uganda (2003) Bulletin of the WHO 81: 799-805. https://doi.org/10.1590/S0042-96862003001100006 27. Chindu C, Mudenda
V and Lucas S. Lung diseases at necropsy in African children dying from
respiratory illnesses: a descriptive necropsy study (2002) Lancet 360: 985-990.
https://doi.org/10.1016/S0140-6736(02)11082-8 28. Schaaf HS, Marais
BJ, Whitelaw A, Hasseling AC, et al. Culture-confirmed childhood tuberculosis
in Cape Town, South Africa: A review of 596 cases (2007) BMC Infectious
Diseases 7: 140. https://doi.org/10.1186/1471-2334-7-140 29.
Yeager
HJ Jr, Lacy J, Smith L and LeMaistre C. Quantitative studies of mycobacterial
populations in sputum and saliva (1967) Am
Rev Respir Dis 95: 998-1004. https://doi.org/10.1164/arrd.1967.95.6.998 30.
Hobby
GL, Holman AP, Iseman MD and Jones J. Enumeration of tubercle bacilli in sputum
of patients with pulmonary tuberculosis. Antimicrob
(1973) Agents Chemother 4:
94-104. https://doi.org/10.1128/AAC.4.2.94 31.
Zar
HJ, Hanslo D and Apolles P. Induced sputum versus gastric lavage for
microbiological confirmation of pulmonary tuberculosis in infants and young
children: a prospective study (2005) Lancet 365: 130. https://doi.org/10.1016/S0140-6736(05)17702-2
Bacteriological Prevalence of Tuberculosis Among Children Seen in Health Facilities in Nasarawa State, Nigeria
Attah Caleb Joseph, Oguche Stephen, Egah Daniel, Banwat Mathilda, Adgidzi Godwin, Nandi Ishaya Tokit
Abstract
Full-Text
Introduction
Materials and Method
Results
Discussion
References