Research Article :
Wondwosen A Matebie,
Wanchang Zhang, Shuo Zhang and Guangbo Xie Background:
Acokanthera schimperia is a medicinal plant, which has been used by
traditional healers as a curative agent in Ethiopia. Objective: The
constituents of the essential oil, which was extracted from the leaves of A.
schimperia, were investigated, and its antibacterial and antifungal
activities were studied. Materials and Methods: The essential oil was
extracted by an ordinary steam distillation process, and its chemical
constituents were analyzed by Gas Chromatography-Mass Spectrometry (GC-MS).
Antibacterial and antifungal activities of the oil were studied by
micro-dilution method against Escherichia coli, Bacillus subtilis (for
bacteria), and Candida albicans (for fungus) respectively. Result: From
the essential oil, 21 compounds were recognized, and making up 56.06 %.
However, the essential oil doesnt show any antimicrobial activities. Conclusion:
This is the first research on A. schimperia concerning its essential
oil and antimicrobial activities. Plants have been used by
Traditional healers for centuries as a source of curative agent, and it has
been spread-out globally and is gaining fame [1]. In Ethiopia, about 80% of the
people, especially those who are living outside the cities are still dependent
on traditional medicines [2,3]. Among different Ethiopia plants, Acokanthera schimperia (A. DC.), which
belongs to family Apocynaceae, is a well-known African arrow poison plant and
dispersed in Eritrea, Ethiopia, Tanzania, West Uganda, Rwanda and Eastern DR
Congo. Outside Africa, It is also found in southern Yemen [4]. In Ethiopia, it
is a tree of dry woodland, thickets, and grasslands in Dry and Moist
agro-climatic zones in nearly all regions [5]. A.
schimperia is a plant locally called “መረንዝ”, which means “Toxic” used for the
preparation of arrow poison in East Africa. It is either used on its own or
mixed with other plants or animal parts. The bark, wood, and roots are the
usual ingredients for arrow poison, and they are also used for suicide and
homicide. The poison from this plant is also used for hunting wild animals and
stray dogs from fields and homes [6]. The leaves and bark are used to treat
different disease and shows antiviral activity in the cases of skin disorders
caused by viruses, and antimalarial activity [7]. In addition, Ethiopian
traditional healers used this indigenous plant for the treatment of epilepsy,
amnesia, eye disease, syphilis, rheumatic pain, elephantiasis, scabies,
leprosy, wound, eczema and warts [8]. Essential oils are combinations
of volatile constituents that present at little amount in the plant, and used
as flavor and fragrances agent in food, pharmaceutical and perfumery industries
[9]. In this paper, we will focus on the essential oil from the leaves of A. schimperia, including its chemical
constituents and bioactivities. Plant
material In September 2015, the leaves of A. schimperia were collected from a
well-known monastery called Debre Libanos which is 90km far from Addis Ababa,
the capital city of Ethiopia and the species of the plant was identified by
Amare Seifu Assefa, a botanist from the Ethiopian Biodiversity Institute. Extraction
of essential oil 200 g coarsely grinded leaves of A. schimperia were socked in 350 mL tap
water for 12 hrs and transferred to 3L volume Clevengers apparatus (flask).
Then 1.5 L distilled water was added to maintain the level of water above the
sample. After that the sample was subjected to steam distillation process for
eight hrs. The distillates were saturated with NaCl and extracted by diethyl
ether. The organic phase was dried by anhydrous Na2SO4 as
water absorbent agent. Diethyl ether was removed from the oil by putting it at
open air until the essential oil remains. Finally, the essential oil was stored
at 4 °C in refrigerator for further use. Analysis
of essential oil The investigation of the
essential oil were done by an Agilent 6890 gas chromatograph interfaced with an
Agilent 5973N mass spectrometer engaged with an HP-5MS capillary column (30 m ×
0.25 mm, 0.25 μm film thickness). The condition for Gas Chromatography (GC):
the temperature programmed at 70 °C for the first 2 minutes, increased
sequentially with a rate of 5 °C/minute until it reaches 300 °C. After that,
the temperature was set to remain constant for the next 2 minutes; The injector
temperature and volume were set at 250 °C and 1μL respectively; the carrier gas
was Helium with flow rate 1 mL/min under split ratio of 1:20; Mass Spectrometry
(MS) condition: EI ionization mode, 70eV, scan range 30-500 Amu, ion spring
temperature was 230°C. Each constituents mass spectra were compared with the
spectrometer database (NIST 11). For quantification purposes, relative area
percentages were used without the use of correction factors. Antimicrobial
activities Escherichia
coli (ATCC 25922) and Bacillus subtilis (ATCC 6633) were used for evaluation of
antibacterial activities and Candida
albicans (ATCC 60193) was used for antifungal evaluation. The bioassays
tests were made in 96-well decontaminated micro-plates using a micro-dilution
method [10, 11]. E. coli and B. subtilis were cultured for 18-hrs and
added to Lysogeny Broth (LB) medium (1 L water, 10 g tryptone, 5 g yeast, and
10 g NaCl) to attain 1 × 105 CFU/ml, and C.
albicans was grown for 4-days and added to Potato Dextrose Broth (PDB)
medium (potato 20%, glucose 2%) to get 1 × 103 spores/mL. The test samples were
dissolved by Dimethyl Sulfoxide (DMSO) to attain from 0.5 to 512 μg/mL
concentration range, which were made by 2-fold sequential dilution method. The
wells holding test strains and diluted samples were incubated for 24 hrs in
isothermal condition (37 °C) for antibacterial bioassay. With the same
condition for antifungal bioassay, the sample was incubated for 4 days at 28
°C. The wells which contain a culture suspension and DMSO were set as negative
controls. Kanamycin and Nystatin were set as positive controls for bacteria and
fungi respectively. All tests were performed twice. The Minimal Inhibitory
Concentration (MIC) was well-thought-out as the lowest antibiotic concentration
that indicates a total production inhibition for the tested microorganisms. The essential oil extracted from
the leaves of A. schimperia gave pale
yellow oil (yield 0.007 % w/w). The constituents of the essential oils with
their retention time and relative percentages were given in Figure 1 and Table 1.Essential Oil and Its Antimicrobial Activity from Ethiopian Acokanthera schimperia
Abstract
Full-Text
Introduction
Materials
and Methods
Results
and Discussion
To View Figure 1, click below
Figure 1: GC-MS profile of essential oil from A. schimperia.
To View Table 1, click below
Table 1: Chemical components (%) of the essential oil from A. schimperia.
A total of twenty-one compounds were identified
representing 75.52 % of the total essential oil of A. schimpera, and damascenone (14.83 %), dihydroactinidiolide
(10.87 %), kaur-16-ene (5.74 %) and (E)-2-tridecenal(5.51 %) were the main
components. Sesquiterpenoids, including six compounds, were found as major in
the oil and represented 32.98 % of the total identified components. The
essential oil extracted from the leaves of A.
schimperia was tested for its antibacterial activity against E. coli (Gram-negative) and B. subtilis (Gram-positive), and its
antifungal activities against C. albicans
(fungi). The results as shown in Table 2
indicated that the essential oil from A.
schimperia did not show any antimicrobial activities.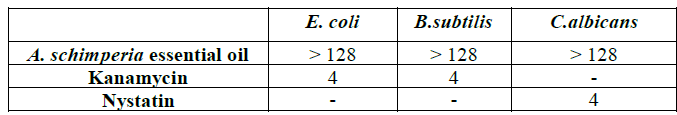
Table 2: Antimicrobial activities of the essential oil (MIC: μg/ml).Conclusion
This report shows that the essential oil of A. schimperia is rich in terpenoids. However, the oil was not found to show any significant antimicrobial activities against E. coli, B. subtilis and C. albicans. Nevertheless, this is the first report on the essential oil of A. schimperia. According to literatures, A. schimperia can be found an attractive medicinal plant. Although, several research works have been done on some plants of this genus till to date, but a large number of this plant is still chemically or pharmacologically unknown. Consequently, a broad future research remains possible in which the isolation of new active principles from A. schimperia would be a great scientific worth.
1. Galen E. Traditional herbal medicines worldwide, from reappraisal to assessment in Europe (2014) J Ethnopharmacol 158: 498-502. https://doi.org/10.1016/j.jep.2014.07.013
2. Kassaye KD, Amberbir A, Getachew B and Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia (2006) Ethiop J Health Dev 20: 127-134. http://dx.doi.org/10.4314/ejhd.v20i2.10023
3. Giday M, Teklehaymanot T, Animut A and Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia (2007) J Ethnopharmacol 110: 516-525. https://doi.org/10.1016/j.jep.2006.10.011
4. Leeuwenberg AJM. Apocynaceae. In: Flora of Ethiopia and Eritrea (ed) Hedberg I, Edwards S and Nemomissa S (2003) The National Herbarium, Addis Ababa University, Addis Ababa, Ethiopia and Uppsala, Sweden, Europe 87-88.
5. Schmelzer GH and Gurib-Fakim A. Plant Resources of Tropical Africa 11(1). Medicinal plants 1 (2008) PROTA Foundation, Wageningen, Netherlands/Backhuys Publishers, Leiden, Netherlands/CTA, Wageningen, Netherlands 34-35.
6. Kupich FK. Studies on African Apocynaceae: the genus Acokanthera (1982) Kew Bulletin 37: 41-67. http://dx.doi.org/10.2307/4114719
7.Gebre-Mariam T, Neubert R and Schmidt PC. Antiviral activities of some Ethiopian medicinal plants used for the treatment of dermatological disorders (2006) J Ethnopharmacol 104: 182-187.https://doi.org/10.1016/j.jep.2005.08.071
8. Mohammed T, Erko B and Giday M. Evaluation of antimalarial activity of leaves of Acokanthera schimperi and Croton macrostachyus against Plasmodium berghei in Swiss albino mice (2014) BMC Complem Altern Med 14: 314. https://doi.org/10.1186/1472-6882-14-314
9. Maffei ME, Gertsch J and Appendino G. Plant volatiles: production, function and pharmacology (2011) Nat Prod Rep 28: 1359-1380. https://doi.org/10.1039/c1np00021g
10. Zhou H, Zhao L, Li W, Yang Y, Xu L, et al. Anti-Mycobacterium tuberculosis active metabolites from an endophytic Streptomyces sp. YIM65484 (2015) Rec Nat Prod 9: 196-200.
11. Dong JW, Cai L, Xiong J, Chen XH, Wang WY, et al. Improving the antioxidant and antibacterial activities of fermented Bletilla striata with Fusarium avenaceum and Fusarium oxysporum (2015) Process Biochem 50: 8-13. https://doi.org/10.1016/j.procbio.2014.09.008
Corresponding author:
Xie G, University of Electronic Science and Technology of China, Chengdu, China, Tel: +86-28-83208238, E-mail: gbxie@uestc.edu.cn
Matebie AW, Zhang W, Zhang S and Guangbo Xie. Essential oil and its antimicrobial activity from ethiopian Acokanthera schimperia (2019) Edelweiss Appli Sci Tech 3: 1-3
Acokanthera schimperia, Essential oil, GC-MS, Antimicrobial activity.