Research Article :
Gosar A, Jadhav S, Patil V,
Folane S, Jadkar A and Vispute T Particle
size is a key factor for modern drug quality since it affects the
bioavailability and dissolution profile of the drug product. Study for Particle
size is helpful to optimize drug product development process and improve the
quality of drugs. In Order to determine the particle size of Glimepiride a
novel and accurate Particle size determination method has been developed for
the determination of particle size distribution of Glimepiride was described in
this paper. This method has shown good
reproducible results. By using water as dispersant wet method is developed and
validated as per International conference on Harmonization guidelines (Q2 (R1))
and found out robust and reproducible with % RSD of d (10), d (50) and d (90)
values found within acceptance limit ranges from 6.05% to 21.84% for d(0.1), 3.04% to 9.87%
for d(0.5) and 4.69% to 14.32% for d(0.9) in validation. The described method
is accurate and validated and successfully applied for the determination of
particle size distribution of Glimepiride. Particle size method is discussed in
detail to ensure in-depth understanding of particle size distribution and
particle size method performance during lifetime of the product. Glimepiride is a white to
yellowish-white, odorless powder and is practically insoluble in water.
Glimepiride acts as an insulin secretagogue and it lowers blood sugar by
stimulating the release of insulin by pancreatic beta cells and by inducing
increased activity of intracellular insulin receptors [1]. Glimepiride
chemically, is 3-ethyl-4-methyl-N-[2-[4-[(4-methylcyclohexyl)carbamoyllsulfamoyl]
phenyl] ethyl]-2-oxo-5H-pyrrole-1-carboxamide is a third generation
sulfonylurea derivative which is commonly used in the treatment of non-insulin
dependent Type 2 diabetes mellitus [2-5]. Glimepiride, marked under the trade
name Amaryl, is the first line medication for the treatment of type-2
diabetes mellitus [6-8]. Relative bioavailability,
extended-released along with bioequivalence and
immediate released is related to particle size of Glimepiride [9-12].
Literature survey done for Particle size distribution method for Glimepiride
and found that particle size determination method is not available. Therefore,
study was carried out to develop a method to determine particle size
distribution of Glimepiride by Particle size analyzer and further validation of
the method, was carried out. Triton x-100, water and
Glimepiride was obtained from Indoco Remedies Ltd, Navi Mumbai, India. Particle size analyzer system of
make Malvern and model is 2000 consists of Dry Scirocco 2000 and wet Hydro2000S accessories. The main goal was to develop
method to obtain the most stable, reproducible, reliable method. Various
dispersant were tried for the Glimepiride on the basis of solubility. Water is
found well suitable dispersant to develop method for determination of particle size distribution
of Glimepiride. Various trial were taken during method development as
mention below, Glimepiride
solubility within a suitable solvent system a critical role in particle
size wet method development since sample preparation is play vital role. Make Malvern Mastersizer, Model
Mastersizer 2000, Dispersion Unit Scirocco 2000, Method parameters are kept as
Particle Refractive index is 1.520, Absorption is 0.1, Analysis Model General
purpose, Obscuration range is 1% to 6%, Dispersive Air pressure is 2.0 bar,
Vibration feed rate is 40%, Sensitivity is Normal, Sample measuring time 5
seconds, Background measuring time 5 seconds, sample preparation is Transferred
about 1 to 2 g of sample into the sample tray with the help of a cleaned
spatula and carried out the analysis The obscuration values are well within the
limit and also weighted residual is less than 1%. Hence, proceeded for next
trial by changing the feed rate 45% to check the effect on particle size.
Various trials were taken by changing instrumental parameters. The obtained
results of d(10), d(50) and d(90) values are not reproducible and % RSD is also not well within general criteria of USP,
General Chapter <429>. Hence next development was carried out by wet
analysis. Method parameters for wet
analysis is Equipment Malvern Mastersizer, Model Mastersizer 2000, Sample
handling unit is Wet Dispersion Unit, Sample model is Hydro 2000S, Dispersant
name is Water, Dispersant refractive index is 1.330, Sample refractive index is
1.520, Sample absorption is 0.1, Sample measurement time is 10 second, Measurement
Snaps is 10,000. Background Measurement time is
10 second, Background Snaps 10,000, Obscuration range is 10-30%, Stirrer
speed is 2000 rpm, No. of measurement cycle 03,sample preparation is Weighed
200.26 mg of sample and transferred in beaker added 1-2 drops of Triton x-100
and few drops of dispersant and make a paste by using glass rod, then added 20
mL of dispersant. Sonicate externally for 40 seconds to form homogeneous
solution. Sample solution was added in the dispersion unit when “Add sample
under Obscuration” message was shown by the instrument software and performed
the analysis. All method parameters were kept
as per above only changed in sonication time 50 Sec. and performed the
analysis. The obscuration is well within limit it is proved that there is no
any effect of sonication time on particle size, Hence, preceded for next trial
by changing stirrer speed check the effect on particle size. The obscuration
values are well within the limit, from the obtained results there is negligible
effect on the particle size by decreased in stirrer speed. Hence, Malvern
Mastersizer, Model Mastersizer 2000, Sample handling unit is Wet Dispersion
Unit, Sample model is Hydro 2000S, Dispersant name is Water, Dispersant
refractive index is 1.330, Sample refractive index is 1.520, Sample absorption
is 0.1, Sample measurement time is 10 second, and Measurement Snaps is 10,000.
Background Measurement time is 10
second, Background Snaps 10,000, Obscuration range is 10-30%, Stirrer speed is
1800 rpm, No. of measurement cycle 03. Performed analysis repeatability
with weight 200.68, 200.96 mg and 200.31 mg to check consistency. The obtained
results of d(10), d(50), and d(90) values of analysis in twice are close to
each other, weighted residue is less than 1% and obscuration is found
satisfactory. To check the reproducibility of method three replicate of sample
was analyzed and results were found reproducible & percentage Relative standard
deviation of d(10), d(50), and d(90) is respectively 4.27%, 9.83% and 14.45
%. The validation work was conducted
according to the ICH (International Conference on Harmonization) guidelines
Q2R1. The method validation parameters include Precision, Intermediate
Precision, Robustness
and Batch Analysis. Procedure: Determined the particle size of six precision samples as per above
method and recorded the particle size for d(0.1), d(0.5) and d(0.9) in Table 1. Acceptance criteria: The % RSD d(10),
d(90) particle size values should not be more than 15, d(50) particle size
values should not be more than 10. If the particle size is below 10 µm then the
% RSD of d(10), d(50), and d(90) will be doubled. Procedure:
Determined
the particle size of six Intermediate precision samples as per above method and
recorded the particle size for d(0.1), d(0.5) & d(0.9) particles in Table 2 and similarly, calculated cumulative average and cumulative
percent relative standard deviation for particle size at d(10), d(50) &
d(90) of twelve measurements i.e. six of precision and six of Intermediate
precision. Acceptance criteria: The % RSD d(10),
d(90) particle size values should not be more than 15, d(50) particle size
values should not be more than 10. If the particle size is below 10 µm then the
% RSD of d(10), d(50), and d(90) will be doubled. To view Table 3, click below Procedure: Determined the
particle size of three replicates as per method of analysis, only change the
stirrer speed to 1800 rpm and recorded particle Size for d (0.1), d(0.5) & d(0.9) in Table 3 and similarly, calculated cumulative average and cumulative
percent relative standard deviation for particle size at d(10), d(50) &
d(90) of nine measurements i.e. six of precision and three of Robustness-1. Acceptance criteria: The % RSD d(10),
d(90) particle size values should not be more than 15, d(50) particle size
values should not be more than 10. If the particle size is below 10 µm then the
% RSD of d(10), d(50), and d(90) will be doubled. Robustness-2 (change in stirrer speed to 2200 rpm) Procedure: Determined the particle size of three
replicates as per method of analysis, only change the stirrer speed to 2200 rpm
and recorded the particle Size for d(0.1), d(0.5) & d(0.9) in Table 4 and similarly, calculated
cumulative average and cumulative percent relative standard deviation for
particle size at d(10), d(50) & d(90) of nine measurements i.e. six of
precision and three of Robustness-2. Acceptance
criteria: The % RSD d(10), d(90) particle size values should
not be more than 15, d(50) particle size values should not be more than 10. If
the particle size is below 10 µm then the % RSD of d(10), d(50), and d(90) will
be doubled. Robustness-3 (change
in Obscuration range to 10-20%) Procedure: Determined the particle size of three replicates as per method of
analysis, only change the obscuration range to 10-20% and recorded the particle
size for d(0.1), d(0.5) & d(0.9) in Table 5 and Similarly,
calculated cumulative average and cumulative percent relative standard
deviation for particle size at d(10), d(50) & d(90) of nine measurements
i.e. six of precision and three of Robustness-3. Acceptance criteria: The % RSD d(10),
d(90) particle size values should not be more than 15, d(50) particle size
values should not be more than 10. If the particle size is below 10 µm then the
% RSD of d(10), d(50), and d(90) will be doubled. Robustness-4 (change in obscuration range to 20-30%) Procedure: Determined the particle size of three
replicates as per method of analysis, only changed the obscuration range to 20
– 30 % and recorded the particle size for d(0.1), d(0.5) & d(0.9) in Table 6 and similarly, calculated
cumulative average and cumulative percent relative standard deviation for
particle size at d(10), d(50) & d(90) of nine measurements i.e. six of
precision and three of Robustness-4. Acceptance criteria: The
% RSD d(10), d(90) particle size values should not be more than 15, d(50)
particle size values should not be more than 10. If the particle size is below
10 µm then the % RSD of d(10), d(50), and d(90) will be doubled. Robustness - 5 (change in sample measurement time to 9 seconds
from 10 seconds) Procedure: Determined the particle size of three
replicates as per method of analysis, only changed the sample measurement time
to 3 seconds and recorded the particle size for d(0.1), d(0.5) & d(0.9) in Table 7 and similarly, calculated
cumulative average and cumulative percent relative standard deviation for
particle size at d(10), d(50) & d(90) of nine measurements i.e. six of
precision and three of Robustness-5. Acceptance criteria: The
% RSD d(10), d(90) particle size values should not be more than 15, d(50)
particle size values should not be more than 10. If the particle size is below
10 µm then the % RSD of d(10), d(50), and d(90) will be doubled. Robustness - 6 (Change in sample measurement time to 11 seconds
from 10 seconds) Procedure: Determined the
particle size of three replicates as per method of analysis, only changed the
sample measurement time to 7 seconds and recorded the particle size for d(0.1),
d(0.5) & d(0.9) in Table 8 and
similarly, calculated cumulative average and cumulative percent relative
standard deviation for particle size at d(10), d(50) & d(90) of nine
measurements i.e. six of precision and three of Robustness-6. Acceptance criteria: The
% RSD d(10), d(90) particle size values should not be more than 15, d(50)
particle size values should not be more than 10. If the particle size is below
10 µm then the % RSD of d(10), d(50), and d(90) will be doubled. Method for determination of particle size distribution
of Glimepiride was developed and validated using Laser diffraction
technique. Dry dispersion technique was assesses and found that dry dispersion
technique is not suitable for and wet dispersion was explored during
development trials. Wet dispersant method frozen for determination of particle
size distribution of Glimepiride. In method validation, method was found
précised with % RSD of 10.59% for d(0.1), 8.57% for d(0.5) and 14.32% for
d(0.9). In intermediate precision % RSD obtained
were 6.05% for d(0.1), 3.04% for d(0.5) and 5.95% for d(0.9). Also, Cumulative
% RSD obtained were 8.39% for d (0.1), 6.71% for d (0.5) and 5.12.40% for d
(0.9).Hence, the method considered as rugged. certain parameters were modified
within the allowed range and the results obtained were within the acceptance
criteria and % RSD ranges from 6.22% to 21.84%
for d(0.1), 3.47% to 9.87for d(0.5) and 4-.69% to 12.85% for d(0.9). It
proved method is rugged. All the analytical data of development and
validation has been compiled and found to be satisfactory. Hence, method
developed for the particle size method can be suitably used for analysis of
Glimepiride active pharmaceutical ingredient. Acknowledgement The Authors wish to extend their gratitude
to Indoco Remedies Ltd. For providing all kind of support. The Author wish to
thank all our colleagues who provided technical assistance during research work
and during compiling data. 1.
Davis
SN. The role of glimepiride in the effective management of Type 2 diabetes
(2004) J Diabetes Complicat 18: 367-376. https://doi.org/10.1016/j.jdiacomp.2004.07.001 2.
Hamaguchi
T, Hirose T, Asakawa H, Itoh Y, Kamado K, et al. Efficacy of glimepiride in
type 2 diabetic patients treated with glibenclamide (2004) Diabetes research
and clinical practice 66: 129-132. https://doi.org/10.1016/j.diabres.2003.12.012 3.
https://www.nlm.nih.gov/medlineplus/druginfo/meds/a696016.html 4.
United
States Pharmacopeia, USP41–NF36. Olopatadine Hydrochloride ophthalmic solution (2018). 5.
Nissen
SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, et al. Comparison of pioglitazone
vs glimepiride on progression of coronary atherosclerosis in patients with type
2 diabetes: the PERISCOPE randomized controlled trial (2008) JAMA. 299: 1561-1573.
https://doi.org/10.1001/jama.299.13.1561 6.
Bando
K and Yamada Y. Glimepiride (Amaryl): a review of its pharmacological and
clinical profile. Nihon yakurigaku zasshi (2001) Folia pharmacologica Japonica
118: 59-67. https://doi.org/10.1254/fpj.118.59 7.
https://www.ich.org/products/guidelines.html 8.
Davis
SN. Insulin, oral hypoglycemic agents and the pharmacology of the endocrine
pancreas. Brunton LL, Lazo JS, Parker KL (ed) (2005) In: Goodman & Gilmans:
The pharmacological basis of therapeutics, McGraw-Hill, New York, USA. 9.
Bonfilio
R, Pires SA, Ferreira LM, de Almeida AE, Doriguetto AC, et al. A discriminating
dissolution method for glimepiride polymorphs (2012) J pharmaceutical sci 101:
794-804. https://doi.org/10.1002/jps.22799 10. Chandiran IS, Kumar BP and Jayaveera KN.
Design and development of microparticulate drug delivery system of Glimepiride
for controlled release (2013) Drug Invention Today 5: 235-240. https://doi.org/10.1016/j.dit.2013.06.006 11. Karkhanis VV and Captain AD. Development
and validation of a liquid chromatographic method for estimation of glimepiride
in tablet dosage form (2013) Int J Pharm Sci Res 4: 2742-2745. Kiran
T, Shastri N, Ramakrishna S and Sadanandam M. Surface solid dispersion of
glimepiride for enhancement of dissolution rate (2009) Int J Pharm Tech Res 1:
822-831. Jadhav S, Indoco
Remedies LTD R&D Centre, Rabale, Navi Mumbai, India,
Tel: +9109869438049, E-mail: shivajij@indoco.com Gosar
A, Jadhav S, Patil V, Folane S, Jadkar A, et al. Development and
validation of new analytical method for the determination of particle size
distribution in glimepiride using laser based particle size analyzer (2019)
Edelweiss
Appli Sci Tech 3: 4-7 Glimepiride, Method development, Method validation,
Particle size analyzerDevelopment and Validation of New Analytical Method for the Determination of Particle Size Distribution in Glimepiride Using Laser Based Particle Size Analyzer
Abstract
Full-Text
Introduction
Materials
and Reagent
Reagents
Instrumentation
Results
and Discussion
Analytical method development
Method validation
Method Validation Parameters
Method precision
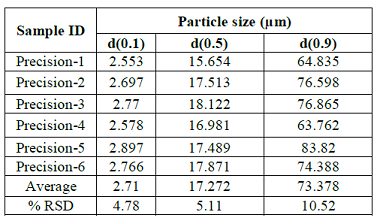
Table 1: Method Precision.Intermediate precision
To view Table 2, click below
Table 2: Intermediate precision.
Table 3: Robustness-1 (Change in stirrer speed to 1800 rpm).
RobustnessRobustness-1 (change
in stirrer speed to 1800 rpm)
To view Table 4, click below
Table 4: Robustness-2 (Change in stirrer speed to 2200 rpm).
To view Table 5, click below
Table 5: Robustness-3 (Change in Obscuration range to 10-20%).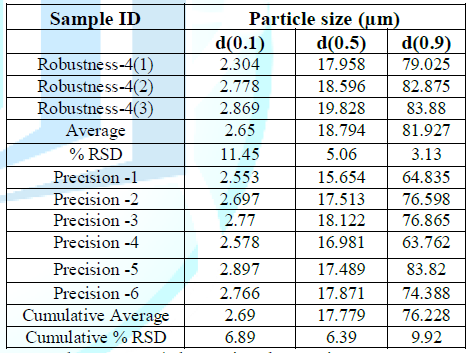
Table 6: Robustness-4 (Change in Obscuration range to 20-30%).
To view Table 7, click below
Table 7: Robustness - 5 (Change in sample measurement time to 9 seconds from 10 seconds).Conclusion
To view Table 8, click below
Table 8: Robustness - 6 (Change in sample measurement time to 11 seconds from 10 seconds).References
*Corresponding
author:
Citation:
Keywords