Research Article :
Main
objective of this article is to review and evaluate recent red cell variant
studies for protection against malaria and natural selection. Malaria is a
parasitic disease highly widespread in tropical and subtropical regions of the
world. It is also one of the leading causes of death worldwide and genes
involved in malaria resistance are the most important for natural selection in
human populations. Multiple red cell variants, which evolved probably to
counter the lethal effects of malaria and confer protection against malaria
through different mechanisms, show high frequencies in malaria endemic
vulnerable populations. Different natural protective/resistance mechanisms
including hampering of parasite growth, invasion related immunological
responses or rapidly elimination of malaria parasite from the infected
erythrocytes of host have briefly been discussed, evaluatedand reviewed.
Conclusions drawn have been projected here. High frequency of inherited hemoglobin
disorders including thalassemiasand red cell G6PD enzyme deficiency, which
seemed to evolve simultaneously in relation to malariaand high mortality caused
by Plasmodium falciparum malaria in different vulnerable populations of
tropical and subtropical parts of world, confirm that the natural selection is
certainly operating against malaria in one way or another; and human population
genetics have distinctly played a significant role in the co-evolution of host
and malaria. The inverse relationship between sickle cell trait and G6PD
deficiency and vice versa, revealed by allele frequencies distribution shown in
our previous studies, is a testimony of disequilibrium, as sickle cell allele
being replaced by G6PD deficiency allele in populations of central India.
Positive natural selection plays a definite role against malaria for
maintaining balance in high frequency endemic populations. There
is a need to recognize and respect the fact that each person is a unique in
this world with its gifted qualities and genetic endowments. Every human-being
fortunately or unfortunately needs to be aware of the fact that the present
human body shape, size and figure is going to give up one day or another day
and what will stay in the world, are the novel deeds, enlightening thoughts,
genuine and truthful contributions and distinguished achievements during the
life span. Human health and disease are the relative terms which depend on the
healthy and balanced up keep against the odd surrounding conditions. When the
human populations are subject to dwelling in varied geography, ecology,
different environmental conditions, climatic fluctuations and changes, disease
susceptibility, persistence of diseases, or bio-social inherent pressures, the
natural selection may alter the genetic allele
frequencies in one population relative to another [1,2]. Positive natural
selection the phenomenon that accounts for the increase in the prevalence of
advantageous traits in a population has played an important role in human
development and evolution as a species. Large differences in allele frequencies
between populations, thus, are the signals in the genome that have undergone
selection. Other
signals of recent positive selection include the long haplotypesand reduced
allelic variation in the regions around the selected variants. Therefore, the
characterization of signatures of positive selection in genes, that are of
adaptive significance in human populations, have greater medical relevance for
identifying the functionally significant variants that play important roles in
health and disease scenario of the host [1,2]. Moreover,
different persons may differ in genetic constitution and its response to an
infectious disease for example, the malaria. Malaria has been one of the most
prevalent and successful parasitic diseases widely spread throughout the globe.
Plasmodium parasites, due to multiple factors, have complex biology, high polymorphism,
increasingly high resistance to anti-malarial drugs in many endemic regions of
the world. As a result of interaction between malaria parasites and human
species have led to fixation of several inherited alterations in many
populations so that some of the underlying mechanisms confer protection against
malaria [1,2]. Natural selection supports such positively involved struggle for
existence of the fittest of all. Malaria
is a parasitic disease highly widespread in tropical and subtropical regions of
the world. This disease is most commonly found in poor countries, having less
developed systems for health infrastructureand inadequate control and
comprehensive preventive strategies. High rates of morbidity and mortality can
be attributed to the lack of timely available diagnostic facilities, due to
financial constraintsand geographical and transport barriers, access to
effective treatment due to insufficient supply of quality medicineand the
growing parasite resistance to anti-malarial drugs such as chloroquine,
pyrimethamine, etc. The immune response induced in humans by parasitic
infection of malaria is a complex one and it varies depending on genetic
make-up of the host, age, epidemiological
factors, level of malaria endemicity, parasite stage, parasite species,
availability of diagnostic facilities and quality of treatmentand repeated
parasitic infection. Both innate and acquired immunity processes are invoked
vigorously during the infection. The
World Malaria Report 2017 presents a comprehensive state of play in global
progress in the fight against malaria up to the end of 2016. In 2016, an
estimated 216 million cases of malaria occurred worldwide. Most malaria cases
in 2016 were in the World Health Organization (WHO) African Region (90%),
followed by the WHO South-East Asia Region (7%) and the WHO Eastern
Mediterranean Region (2%) and the like. Plasmodium falciparum is the most
prevalent malaria parasite in sub-Saharan Africa, accounting for 99% of
estimated malaria cases in 2016. In 2016, there were an estimated 445,000
deaths from malaria globally. About 2 million confirmed malaria cases and 1,000
deaths are reported annually, although 15 million cases and 20,000 deaths are
estimated by WHO South East Asia Regional Office. India contributes 77% of the
total malaria in Southeast Asia. India was fourth with 7 percent of deaths,
after Nigeria (30%), the Democratic Republic of Congo (14%), Burkina Faso (7%) and
so on [3]. Malaria
is one of the leading causes of death worldwide and genes involved in malaria
resistance are the most important for the natural selection in human
populations. In 1949, Haldane suggested that infectious disease could be a
strong selective force in human populations [4]. Evidence for the strong
selective effect of malaria resistance includes the high frequency of a number
of detrimental hemato-genetic
diseases (including the different hemoglobinopathies, thalassemiasand red cell
enzymopathy), caused by the effects of these malaria resistance variants. In
view of this pathetic scenario, it is justifiable that population genetics
could be useful to determine the amount and pattern of natural selection in
human population isolates [5-12]. Red
cell variants that modulate malaria risk can serve as models to identify
clinically relevant mechanisms of pathogenesisand thus define parasite and host
targets for next-generation therapies. Multiple red cell variants are known to
confer protection from malaria. From a biological point-of-view, these insights
highlight the co-evolution of host and parasite and serve as a model of
balancing selection. From a clinical perspective, these relationships represent
a naturally occurring model of protection from severe, life threatening
malaria, which can be used to isolate the mechanisms of parasite pathogenesis.
By preventing malaria parasites from causing disease, these red cell variants could
help discover clinically significant mechanism(s) of pathogenesis and
investigating them as targets for future therapeutics. JBS
Haldane was the first who speculated the Darwinian (Natural) selection that,
depending on the genetic make-up, the people would have a different risk of
dying when they are confronted by a parasitic organism; so much so that, even
if, a gene offering protection against those parasites were, otherwise,
harmful, its frequency would increase when a population was exposed to the
parasites [4,13]. Later, Haldane hypothesized that one important example could
be of thalassemia
in the face of malaria for several reasons. First, one type of malaria, caused
by Plasmodium falciparum, is highly lethal. Second, it is estimated to have
been spread in many parts of the world for several thousands of years, i.e. for
several hundreds of generations; thus, malaria as an agent of natural selection
seemed to be a better candidate than an infectious disease, causing occasional
epidemics, even if associated with high mortality (such as influenza or AIDS).
Third, deaths from malaria take place mostly in children, i.e. before
reproduction, a critical criterion for effective selection. Last but not the
least, Plasmodia
take on different forms in the course of their life cycle, but what causes a
disease, are the intra-erythrocyte parasites. Therefore, in principle, it is
not surprising that, if red cells are in any way abnormal (as they are, for
instance, in thalassemia), they may affect the chance of success of the
parasites [14]. Similarly,
alpha (α+)-thalassemia, being very common in malaria endemic regions, it has
been considered to confer protection against clinical disease caused by severe
forms of Plasmodium falciparum malaria infection. In the same way, beta
(β+)-thalassemia provides protection against the Plasmodium falciparum malaria
with significantly lower growth of malaria parasite inside the infected
erythrocytes and higher phagocytosis of β-thalassemic infected erythrocytes
when compared to normal infected erythrocytes. Moreover, the resistance given
to malaria parasite inside the infected erythrocytes is almost identical to
that of sickle cell trait infected erythrocytes. This
brief review has been focused on the close and complex relationship of blood
disease, e.g. the Sickle Cell Anemia (SCA) with infectious disease of malaria.
Sickle cell anemia is a major hemolytic anemia and its epidemiology represents
a remarkable signature of the past and present world distribution of Plasmodium
falciparum malaria [14]. On one hand, heterozygotes (Hb AS) for the sickle gene
are relatively protected against the danger of dying with malaria as now firmly
established through a number of clinical field studies reviewed from different
parts of Africa, South East Asia, Indian Sub-continentand the Middle East
regions [11,15-20]. In addition, the experimental work is consistent with the heterozygote (Hb AS)
red cells infected with Plasmodium falciparum are preferentially removed by a
mechanism of macrophages [17,19]. On the other hand, patients homozygous for
the sickle gene, suffer from sickle cell anemia,
are highly susceptible to the lethal effects of malaria [14]. The
simplest explanation of this fact is that malaria makes the anemia of
homozygous (Hb SS) cells more severe; leading to often hyposplenism, which
reduces the clearance of parasites. From public health point of view, it is
important that in malaria-endemic countries, the patients with sickle cell
anemiaand particularly the children, are being protected from malaria by
appropriate prophylaxis [14,21,22]. Since the humans, like most animals, are
diploid, therefore, have more options in this respect. Sickle cell anemia is a
disease of homozygotes (Hb SS)-thats why it is called recessive disease-whereas,
heterozygotes (Hb
AS) are normal for most intents and purposes. The first test of Haldanes
hypothesis was carried out by Allison when he showed not only that the S gene
was frequent in areas of high malaria transmission, but also that AS (Hb)
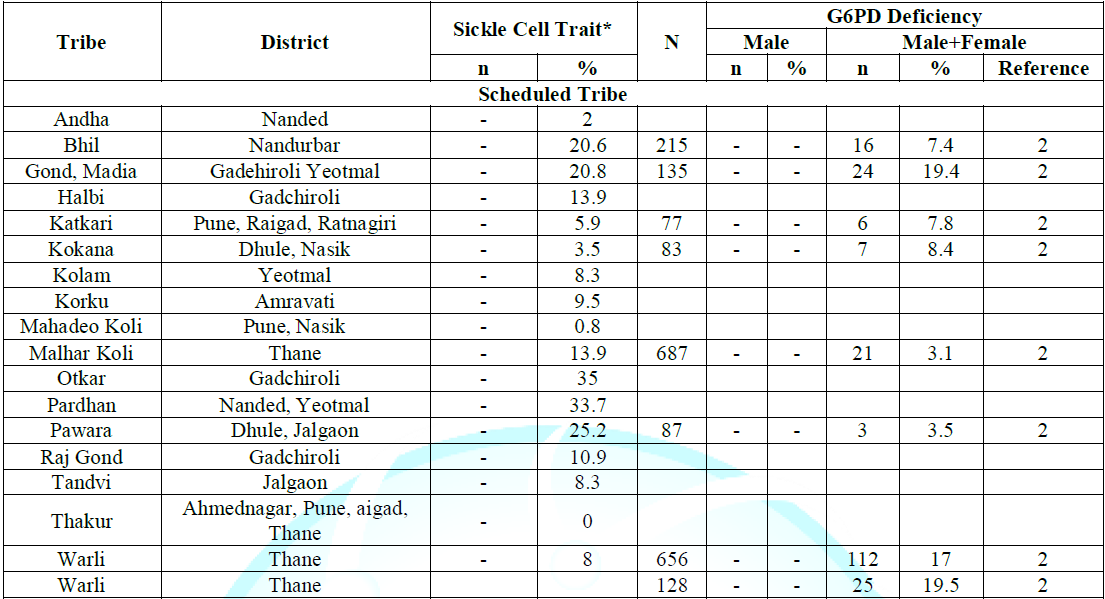
heterozygotes seemed to have less malaria [4,23]. Note: *Data from Reference 2. By
the laws of population genetics it is to be expected that wherever the sickle
(S) gene is common, there will be many patients suffering from sickle cell
anemia, a severe burden in the population [21,24]. However, in the same
population a much larger number of heterozygotes (Hb AS) will have the
advantage of being malaria-resistant. The disadvantage of homozygotes (Hb SS)
coexisting with the advantage of heterozygotes (Hb AS)-therefore called a balanced
[2,16,17]. High fetal and childhood mortality have also recently been described
[37,38]. Among the most relevant mechanisms, reduced erythrocyte invasion
by the parasite, decreased intra-erythrocyte parasite growth enhanced
phagocytosis of parasite-infected erythrocytes and increased immune response
against parasite infected erythrocytes have been described [27,31,39,40]. Thus,
malaria and sickle cell anemia are still major challenges, being the major
public health problems. Patients with sickle cell anemia carry the genetic
burden that has helped human populations to survive in malaria-endemic regions
of the world. The protective effect of the Hb S gene against malaria is one of
the best documented examples in the human species of balanced polymorphism, in which
the severe disease of homozygotes (Hb SS or SCA) is balanced by the advantage
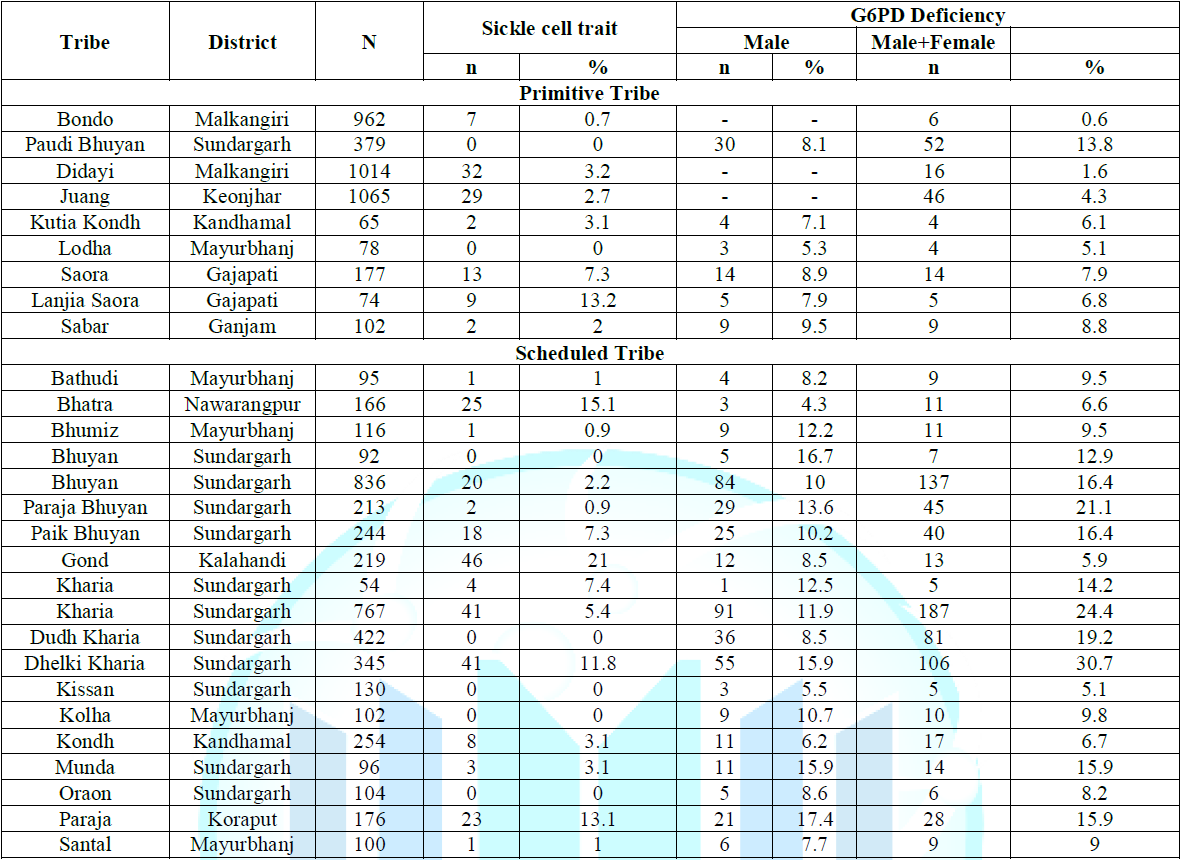
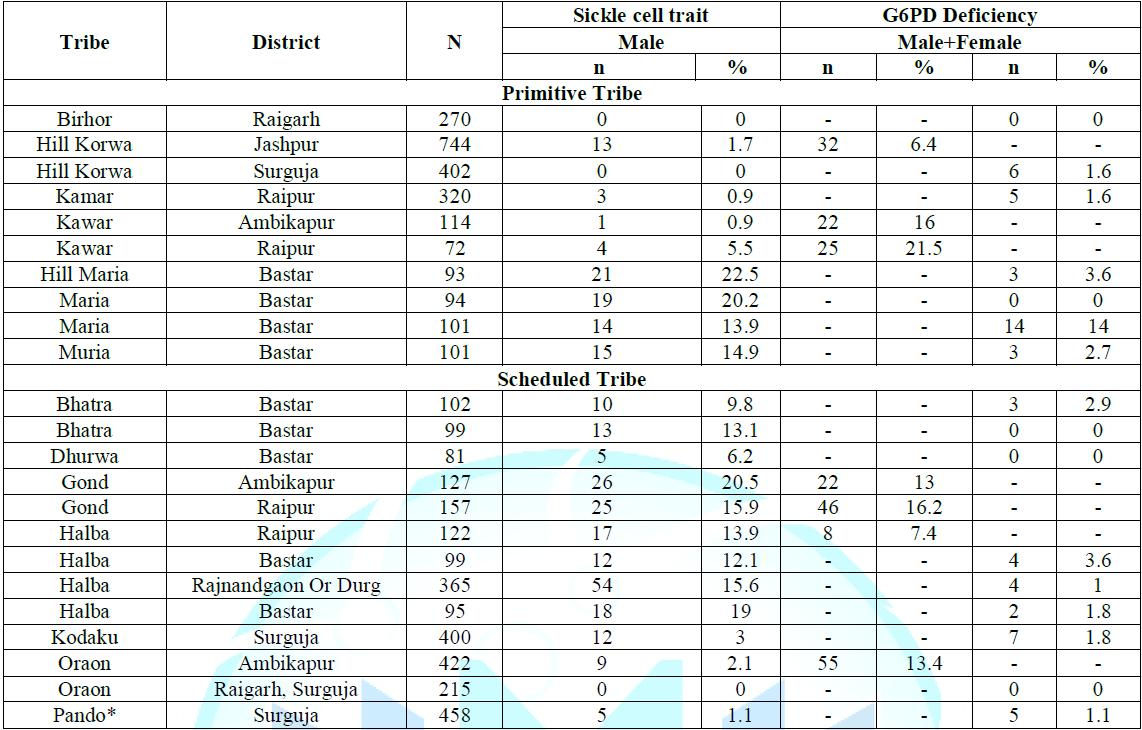
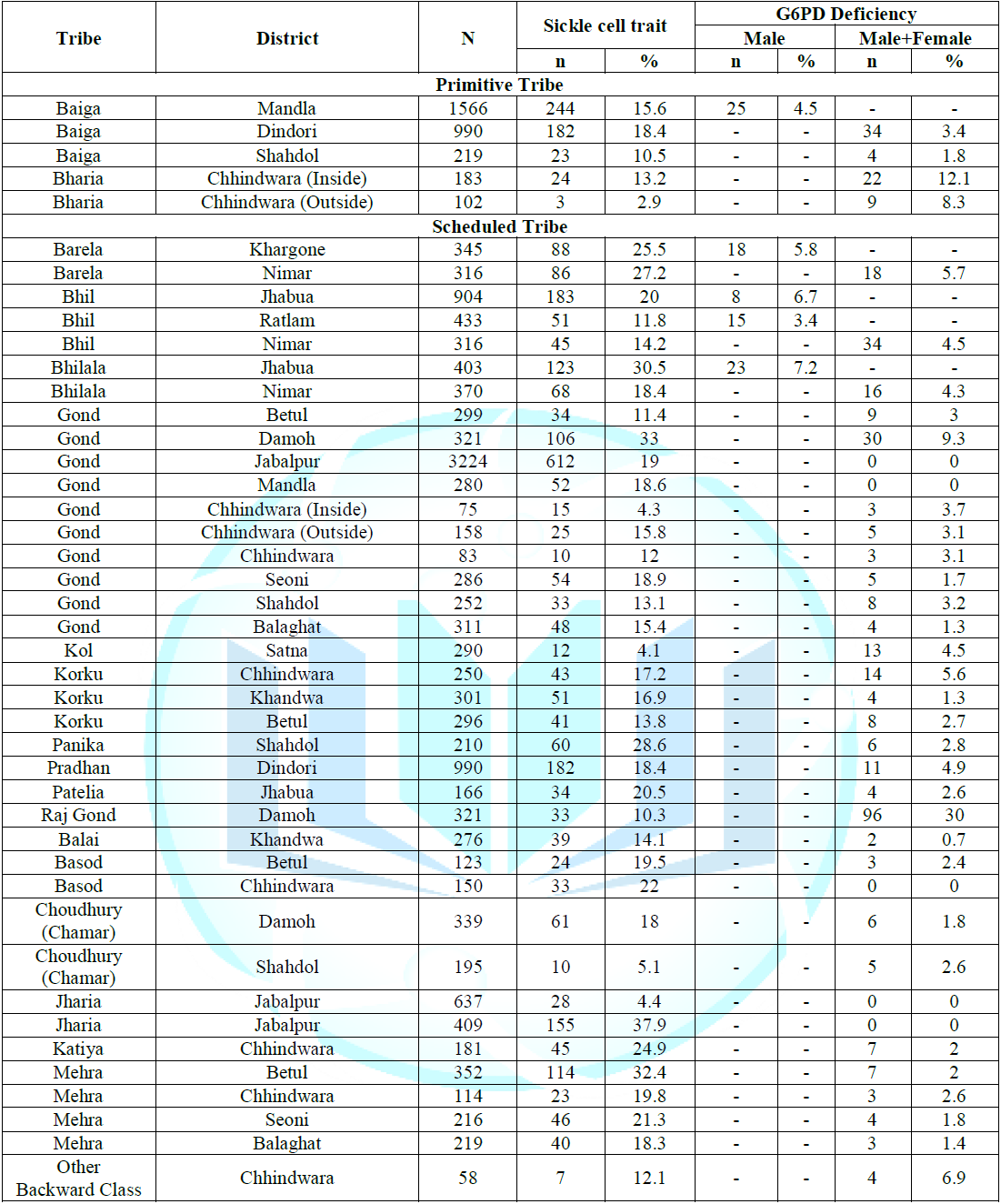
of Hb AS heterozygotes (Tables 1-4). Note: *A case of
hemoglobin AE was detected. **Data from Reference 2. The
hemoglobin variants/mutants, namely, Hb C and Hb S, are known to protect
carriers from severe falciparum malaria. There is a malaria protection-inducing
mechanism, that intra-erythrocyte parasite growth becomes reduced in
individuals having Hb C erythrocytes in both mild and severe malarial
infection. Individuals homozygous for Hb CC display a reduced risk of having
severe or non-severe infection by Plasmodium falciparum malaria [17,41]. Heterozygous
form of hemoglobin E confers protection against severe malarial episodes
because there is reduced erythrocyte invasion by merozoites, lower
intra-erythrocyte parasite growthand enhanced phagocytosis of infected
erythrocytes [42-44]. Hereditary
spherocytosis (also known as Minkowski–Chauffard syndrome) is an abnormality of
erythrocytes. The disorder is caused by mutations in genes relating to membrane
proteins that allow for the erythrocytes to change shape. Both Hereditary
Ovalocytosis/Ellyptocytosis (Band 3 Variant) and Hereditary Spherocytosis
variants reduce the Plasmodium falciparum growth in vitro [45,46]. Ovalocytosis
is an uncommon variant of hereditary ellyptocytosis
belonging to the erythrocyte membrane inherited disorder. Only the
heterozygotic form, which is asymptomatic and endemic in Southeast Asia,
derives its name as Southeast Asian ovalocytosis [41]. It gives protection
against cerebral malaria. The
enzyme G6PD deficiency is a genetic disorder that occurs almost exclusively in
males. This condition mainly affects red blood cells, which carry oxygen from
lungs to various tissues throughout the body, resulting in reduced oxygen flow
to the different organs. This can cause fatigue, yellowing of the skin and eyesand
shortness of breath. Additional symptoms of G6PD deficiency include: ·
rapid
heart rate ·
shortness
of breath ·
urine
is dark or yellow-orange ·
fever ·
fatigue ·
dizziness ·
paleness ·
jaundice,
or yellowing of the skin and whites of the eyes The
G6PD enzyme deficiency results from mutations in the G6PD gene. The mutation
reduces the amount of G6PD enzyme or alters its structure, so that enzyme can
no longer play its protective role. As a result, reactive oxygen species can
accumulate and damage red blood cells. Factors such as infections, certain
drugs, or ingesting fava beans can increase the levels of reactive oxygen
species, causing destruction of erythrocytes faster than the body can replace
them. A reduction in number of red blood cells causes the signs and symptoms of
hemolytic anemia. G6PD enzyme involves in the normal processing of carbohydrates
metabolism. It also protects red blood cells from the effects of potentially
harmful molecules called reactive oxygen species by products of normal cellular
functions. Note: *Data from Reference 2. Chemical
reactions, involving G6PD, produce compounds that prevent reactive oxygen
species from building up to toxic levels within red blood cells. In affected
individuals, a defect in G6PD enzyme causes red blood cells to break down,
called hemolysis, prematurely faster than the body can replace them [30]. Once
G6PD deficiency has progressed to hemolytic anemia, more
aggressive treatment may be required. This usually includes oxygen therapy and
blood transfusion to replenish oxygen and red blood cells. The affected person
will need to stay in the hospital, while receiving these treatments as close monitoring
required of severe hemolytic anemiaand is critical for ensuring a full recovery
without complications. In people with G6PD deficiency, hemolytic anemia is most
often triggered by bacterial or viral infections or by certain drugs (such as
some antibiotics and medications used to treat malaria). Table 4: Distribution of
sickle cell trait and G6PD deficiency in scheduled caste and scheduled tribe
communities of Maharashtra, India. Hemolytic
anemia can also occur after eating fava beans or inhaling pollen from fava
plants (a reaction called favism) [2,9]. It may also be triggered by infections
or by certain drugs such as: · Antimalarials, a
type of medication used to prevent and treat malaria · Sulfonamides, a medication
used for treating various infections · Aspirin, a drug
used for relieving fever, pain and swelling · Some Non-steroidal
Anti-Inflammatory Medications (NSAIDs). Once
the underlying cause is treated or resolved, symptoms of G6PD deficiency
usually disappear within a few weeks. G6PD deficiency is also a significant
cause of mild to severe jaundice
in newborns. Many people with this disorder, however, never experience any
signs or symptoms and are unaware that they have the condition. On
the other hand, age specific mortality is high, i.e., as the age advances, the
number of G6PD deficiency individuals go on decreasing in a malaria endemic
populations, has been reported by some investigators [32]. The G6PD enzyme
deficiency is inherited as an X-linked recessive pattern. The gene associated
with this enzyme deficiency is located on the X chromosome, which is one of the
two sex-chromosomes. In males (who have only one X chromosome, in hemizygous
condition), one altered copy of the gene in each cell is sufficient to cause
the deficiency. In
females (who have two X chromosomes), a mutation would have to occur in both
copies (alleles) of the gene to cause the disease. But, daughters always get
their X chromosomes from parents, one each from father and mother. Therefore,
they may be heterozygote, if father is affected and mother is normal.
Similarly, they may also be heterozygote, if one of the X chromosomes is
carrier or trait for G6PD deficiency. Further it all depends on the X chromosome, either
inherited from affected father or carrier motherand also on the activation of
one (normal or abnormal) out of the mothers two X chromosomes according to the
Lyons Hypothesis [9,10]. Thus, G6PD deficiency would have to occur in both X
chromosomes (counter parts) of females (from father as well as from mother) to
fully express the defective gene (in homozygous state). Males
are affected by X-linked recessive disorders much more frequently than the
females (Tables 1-4). A characteristic of X-linked inheritance is that fathers
cannot pass X-linked traits to their sons [47]. In contrast to the findings by
Ruwende et al. a later study showed that a form of G6PD deficiency confers
protection against severe malaria in its uniform state (hemizygous males and
homozygous females) but not in
its mosaic state, i.e. heterozygous females [48]. This finding is consistent
with those protection mechanisms involving either enhanced phagocytosis or the
effects on pathogenic consequences in the microcirculation of parasitized
erythrocytes, since both are expected to operate preferentially on uniformly
deficient erythrocytes [26]. High
frequency distribution of inherited hemoglobin disorders including thalassemiasand
red cell G6PD enzyme deficiency, which have probably evolved simultaneously in
relation to malaria in different vulnerable and malaria endemic populationsand
high mortality caused by Plasmodium falciparum malaria in different tropical
and subtropical parts of the world confirm that the natural selection is
certainly operating against malaria in one way or another; and human population
genetics play a major role in this process of co-evolution of human-beings and
malaria. The people who have a G6PD deficiency mutation may be partially
protected against Plasmodium falciparum malaria. The deficiency of G6PD enzyme
or a reduction in the amount of functional G6PD appears to make it more
difficult for the malaria parasite to invade red blood cells, inhibits its growth
and phagocytises
rapidly [49]. G6PD deficiency occurs most frequently in areas of the world
where malaria is common. Moreover, disequilibrium of genetic markers such as
various variants of hemoglobin and high occurrence of G6PD deficiency is reflected
as the natural selection mechanism for protection against malaria [1,2,11,27,31]. Natural
selection can maintain deleterious alleles in the population if there is a
heterozygote advantage (positive selection) as in the case of sickle cell trait
(Hb AS). When the frequency of sickle cell allele decreases in malaria endemic
cross-section of the tribal population in India, the frequency of G6PD enzyme
deficiency allele increases and vice versa [1,2]. This trend for an inverse
relationship between sickle cell disorders and G6PD deficiency due to
disequilibrium in major scheduled caste and tribal communities of
Central-Eastern India, is fascinating one [1,2]. This medical aspect is
important from an evolutionary biological background and could be an excellent
starting point for molecular analyses to determine the signature of natural
selection in the genomic regions of the β-globin and G6PD genes. This may
further provide a mechanism for how natural selection operates against malaria
when two mutations occur in the same geographical region [2]. Similarly,
further work on the remarkable epistatic interactions between various
malaria-protective polymorphisms could provide invaluable information about the
mechanisms for the distribution of the different forms of inherited hemoglobin
disorders particularly in high-frequency populations (Tables 1-4). Further
natural selection had played a major role initially in favor of sickle cell, β
-thalassemia and G6PD mutations so that they have probably evolved as a protective
mechanism against the lethal effects of malaria. Since the selection favors the
mutation with least cost to the population, as the clinical manifestations of
G6PD deficiency are mild and do not result in a complete loss of enzyme
activity against the sickle cell disease with high morbidity and mortality in
the regionand predominant frequency of G6PD deficiency over the sickle cell
disorders in some aboriginal communities in India. It seems that the
replacement of the sickle cell allele for G6PD deficiency allele is occurring
due to disequilibrium in the major scheduled castes/tribes of Chhattisgarh,
Madhya Pradesh, Maharashtraand Odisha state in Central India [2]. This means
that the decrease in sickle cell allele is compensated by the increase of G6PD
deficiency alleles. It seems that different abnormal hemoglobin variants
(C,D,E,F,S and thalassemias) and G6PD enzyme deficiency are the directed
mutations against the malaria malady as the heterozygotes or carriers of these
genetic traits do not suffer severely from the dreadful malaria (Tables 1-4). Another
important factor is the relatively high frequency of consanguineous marriages in
many regions of India with high frequency of these red cell genetic variants;
this mechanism has an important effect on increasing the gene frequency of the
fore said recessively inherited disorders in vulnerable populations of central
India [50,51]. Although accurate data on the frequency of consanguineous
marriages are lacking, there is no doubt that this is an important factor in
helping to maintain the global or regional or local health problem posed by the
high frequency of red cell genetic variants and malaria conditionsand
significantly contributing towards the high morbidity, maternal mortalityand
fetal and childhood mortality [21,22,37,38]. The
varying distribution of some of the hemoglobin disordersand G6PD enzyme
deficiency reflects strong founder effects of their original inhabitants in
different populations [6,8]. Another important factor is the epidemiological
conditions, whereby as the public health and nutritional standards improve in
the poorer countries, babies with these red cell hemolytic
conditions who would, otherwise, have died in early life, are now living long
enough to present for diagnosis and management [21]. An estimated 400 million
people worldwide have G6PD deficiency. This condition occurs most frequently in
certain parts of Africa, Southeast Asia including India, the Mediterraneanand
the Middle East. It affects about 1/10 African American males in United States
[17,41]. The
frequency distribution of inherited hemoglobin disorders and Plasmodium
falciparum malaria are posing increased burden on human health resources. Their
high frequency is a reflection of natural selection combined with a high
frequency of consanguineous marriages in many communities and regions, together
with an epidemiological expansion due to public health improvement in the
affected communities as more babies with these disorders survive to present for
treatment in future too. The strongest evidence for Hb S and (α+)- and (β+)-thalassemias,
without any doubt, that malaria is responsible for the current distributions of
all the major hemoglobin disorders in the world. Malaria is one of the leading
causes of death worldwide and has been suggested as the most potent type of
selection in humans in recent millennia. As a result, genes involved in malaria
resistance are excellent examples of strong selection in recent years. Perhaps
best known is the sickle cell hemoglobin variant, which is often used as an
example of heterozygote advantage. In
addition, G6PD deficiency illustrates strong selection at an X-linked locus,
followed by β-globin variants C, D, E and S variants. In 1949, Haldane
initially suggested that infectious disease; specifically the malaria could be
a strong selective force in human populations. Evidence for the strong
selective effect of malaria resistance includes the high frequency of a number
of detrimental genetic diseases caused by the pleiotropic effects of these
malaria resistance variants. In contrast, there are many changes that modify
levels of expression and provide malaria resistance for G6PD deficiency, α-thalassemiaand
β-thalassemia. Malaria parasites have co-evolved with the host and constitute
an important deriving evolutionary force behind common erythrocyte variants
such as thalassemia, sickle cell disease, Hb C, Hb D, Hb E and G6PD deficiency
and other erythrocyte anomalies. Host-parasite
interactions have led to a hosts relative resistance to the parasite. There
could be two reasons for malaria mediated evolutionary selection: · Strong selective
pressure in case of higher frequency of Hb S allele found in malaria exposed
populations; and · Independent
evolutionary responses developed by different populations both at global (e.g.
Hb C, Hb D, Hb E and Hb S confer protection against malaria because mutations
affect the hemoglobin functionality) and local level (four different HbS
haplotypes found in Africa) and the Arab-Indian haplotype is different from the
African haplotypes. Different
mechanisms conferring protection against malaria are widely found in different
populations of the worldand that the populations have evolved and developed different
genetic variants, which are related to resistance to the malaria disease. This
could imply that the maintenance of these alleles in the population has been
due to the effects of positive selection against the lethal malaria. There also
seems to be disequilibrium and competition between two red cell variants, i. e.
Sickle cell disease (Hb SS) and G6PD enzyme deficiency. When the frequency of
sickle cell allele decreases in malaria endemic tribal population in India, the
frequency of G6PD enzyme deficiency allele increases and vice versa. This
trend for an inverse relationship between sickle cell disorders and G6PD
deficiency in major scheduled caste and tribal communities of Central-Eastern
India, is fascinating one. Since the selection favors the mutation with least
cost to the population, as the clinical manifestations of G6PD deficiency are
mild and do not result in a complete loss of enzyme activity against the sickle
cell disease with high morbidity and mortality in the region. Even though the
above could explain, why mutations conferring malaria protection are highly
variable and maintained in the population, the association between sickle cell
disease and G6PD enzyme deficiency seems to be well-suited here. Thus, the
protection is principally present for severe disease and largely absent for
Plasmodium falciparum infection, suggesting that hemoglobin disorders
specifically neutralize the parasites in vivo mechanisms of pathogenesis. These
genetic traits-including Hemoglobin C (Hb C), Hemoglobin D (Hb D), Hemoglobin E
(Hb E), Hemoglobin S (Hb S) and α- and β-thalassemias-are the most common
monogenic human disorders and can confer remarkable degrees of protection from
severe, life-threatening falciparum malaria in African children: the risk is
reduced 70% by homozygous Hb C and 90% by heterozygous Hb S (sickle-cell
trait). These hemoglobin variants thus represent a natural experiment to
identify the cellular and molecular mechanisms by which Plasmodium falciparum
produces clinical morbidity, which remain partially obscured due to the
complexity of interactions between this parasite and its human host. Multiple
lines of evidence support a restriction of parasite growth by various
hemoglobinopathiesand recent data suggest this phenomenon may result from host
micro RNA interference with parasite metabolism. Therefore, owing to the
co-evolution of humans and Plasmodium falciparum parasites, the human genome is
imprinted with polymorphisms that not only confer innate resistance to
falciparum malaria, but also cause hemoglobinopathies to
counter the adverse effects of severe malaria. The
author gratefully acknowledges all the researchers, investigators and authors
whose works have been quoted directly or indirectly in this write up to explain
or interpret the findings logically to make it understandable. 1.
Balgir
RS. Do tribal communities show inverse relationship between sickle Cell disorders
and glucose-6-phosphate dehydrogenase deficiency in malaria endemic areas of
central-eastern India? (2006) Homo J Compar Hum Biol 57: 163-176. https://doi.org/10.1016/j.jchb.2006.01.003 2.
Balgir
RS. Community expansion and gene geography of sickle cell trait and G6PD
deficiencyand natural selection against malaria: experience from tribal land of
India (2012) Cardiovasc Hematol Agents Med Chem 10: 3-13. https://doi.org/10.2174/187152512799201190 3.
World Malaria Report 2017:
WHO Key Points. 4. Haldane JBS.
Disease and evolution (1949) Ricerca Sci 119: 68-76. 5. Balgir RS. Serogenetc studies in the Gypsy Sikligars of North western
India (1986) Hum Biol 58: 171-187. 6. Balgir RS and Sharma JC. Genetic markers in the Hindu and Muslim Gujjars
of North-western India (1988) Am J Phys Anthropol 75: 391-403. https://doi.org/10.1002/ajpa.1330750310 7. Balgir RS, Dash BP and Murmu B. Blood groups, hemoglobinopathy and
G-6-PD deficiency investigations among fifteen major scheduled tribes of
Orissa, India (2004) Anthropologist 6: 69-75. https://doi.org/10.1080/09720073.2004.11890830 8.
Balgir
RS. The spectrum of hemoglobin variants in two scheduled tribes of Sundargarh
district in North-western Orissa, India (2005) Ann Hum Biol 32: 560-573. https://doi.org/10.1080/03014460500228741 9.
Balgir
RS. Genetic burden of red cell enzyme glucose-6-phosphate dehydrogenase
deficiency in two major scheduled tribes of Sundargarh district in
North-western Orissa (2007) Curr Sci 92: 768-774. https://doi.org/10.1080/03014460500228741 10.
Balgir RS. Genetic diversity of hemoglobinopathies,
G6PD deficiency and ABO and Rhesus blood groups in two isolates of primitive
Kharia tribe in Sundargarh district of North-western Orissa, India (2010)J Comm Genet 1: 117-123. https://doi.org/10.1007/s12687-010-0016-y 11.
Millimono
TS, Loua KM, Rath SL, Relvas L, Bento C, et al. High
prevalence of hemoglobin disorders and glucose-6-phosphate dehydrogenase (G6PD)
deficiency in the Republic of Guinea (West Africa) (2012)Hemoglobin 36: 25-37. https://doi.org/10.3109/03630269.2011.600491 12.
Balgir
RS. Spectrum ofhemoglobinopathies
andevaluation of prevalence of
beta-thalassemia trait in the tribal land of middle India (2013) Intl Public
Healt J 5: 165-177. 13.
Haldane JBS. The
causes of evolution (1932) Cambridge University Press, UK.
14.
Luzzatto
L. Sickle cell anaemia and malaria (2012) Mediterr J Hematol Infect Dis 4:
e2012065. https://doi.org/10.4084/mjhid.2012.065 15.
Kwiatkowski
DP. How malaria has affected the human genome and what human genetics can teach
us about malaria (2005) Am J Hum Genet 77: 171-192. https://doi.org/10.1086/432519 16. Lopez C, Saravia C, Gomez A, Hoebeke J and
Patarroyo MA. Mechanisms of genetically- based resistance to malaria (2010)
Gene 467: 1-12. https://doi.org/10.1016/j.gene.2010.07.008 17.
Taylor
SM, Cerami C and Fairhurst RM. Hemoglobinopathies: slicing the Gordian knot of
Plasmodium falciparum malaria pathogenesis (2013) Plos Pathog 9: e1003327 https://doi.org/10.1371/journal.ppat.1003327. 18.
Hedrick PW. Resistance to
malaria in humans: the impact of strong, recent selection (2012) Malaria J 11: 349. https://doi.org/10.1186/1475-2875-11-349 19.
Pathak
V, Colah R and Ghosh K. Effect of inherited red cell defects on growth of
Plasmodium falciparum: an in vitro study (2018) Indian J Med Res 147: 102-109. https://doi.org/10.1016/j.gene.2010.07.008 20.
Goldsmith
JC, Bonham VL, Joiner CH, Kato GJ, Noonan AS, et al. Framing the research agenda for sickle cell trait: building on the
current understanding of clinical events and their potential implications
(2012) Am J Hematol 87: 340-346.
https://doi.org/10.1002/ajh.22271 21.
Balgir RS. Public health challenges of
hemoglobinopathies in tribal land of India: a necessity of introducing genetic
services in the health care systems approach (2014) Br Biomed Bullet 2: 489-503. 22. Balgir RS. Hematological profile of hemoglobinopathies in maternal health
and reproductive outcome in pregnant mothers at a tertiary hospital in central
India (2018) J Hematol Multiple Myeloma 3: 1012. 23.
Allison AC.
Protection afforded by the sickle cell trait against subtertian malarial
infection (1954) Br Med J 1:
290-294. http://dx.doi.org/10.1136/bmj.1.4857.290 24.
Balgir RS. Prevalence of hemolytic anemia and
hemoglobinopathies among the pregnant women attending a tertiary hospital in
central India (2015) Thalassemia Reports 5: 16-20. https://doi.org/10.4081/thal.2015.4644 25. Olumese
PE, Adeyemo AA, Ademowo OG, Gbadegesin RA, Sodeinde O, et al. The clinical
manifestations of cerebral malaria among Nigerian children with the sickle cell
trait (1997) Ann Trop Paediat
17: 141-145. https://doi.org/10.1080/02724936.1997.11747877
26. Luzzatto
L and Pinching AJ. Commentary to R Nagel -Innate Resistance to Malaria: The
Intra-erythrocytic Cycle (1990) Blood Cells 16:
340-347. 27.
Ayi K, Turrini F,
Piga A and Arese P. Enhanced phagocytosis of ring-parasitized mutant
erythrocytes: a common mechanism that may explain protection against falciparum
malaria in sickle trait and beta-thalassemia trait (2004) Blood
104: 3364-3371. http://dx.doi.org/10.1182/blood-2003-11-3820 28.
Luzzatto L,
Nwachuku-Jarrett ES and Reddy S. Increased sickling of parasitised erythrocytes
as mechanism of resistance against malaria in the sickle-cell trait (1970) Lancet
1: 319-321. http://dx.doi.org/10.1016/S0140-6736(70)90700-2
29.
Luzzatto L.
Genetics of red cells and susceptibility to malaria (1979) Blood
54: 961-976. 30. Balgir RS. Ethnic and regional variations in the red cell
glucose-6-phosphate dehydrogenase deficiency in India (1989) Indian J Hematol 7:
101-109. 31.
Cappadoro
M, Giribaldi G, OBrien E, Turrini F, Mannu F, et al. Early phagocytosis of
glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by
Plasmodium falciparum may explain malaria protection in G6PD deficiency (1998)
Blood 92: 2527-2534. https://doi.org/10.1182/blood.v92.7.2527.2527_2527_2534 32. Balgir RS, Mishra RK and Murmu B. Clinical and hematological profile of
hemoglobinopathies in two tribal communities of Sundargarh district in Orissa,
India (2003) Intl J Hum Genet 3: 209-216. https://doi.org/10.1080/09723757.2003.11885854 33. Cabrera
G, Cot M, Migot- Nabias F, Kremsner PG, Deloron P, et al. The sickle cell trait
is associated with enhanced immunoglobulin G antibody responses to Plasmodium
falciparum variant surface antigens (2005) J Infect Dis 191: 1631-1638. https://doi.org/10.1086/429832
34.
Cholera R,
Brittain NJ, Gillrie MR, Lopera-Mesa TM, Diakite SA, et al. Impaired
cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle
hemoglobin (2008) Proc Natl Acad Sci 105:
991-996. http://dx.doi.org/10.1073/pnas.0711401105
35.
Adeloye A,
Luzzatto L and Edington GM. Severe malarial infection in a patient with
sickle-cell anaemia (1971) Br Med J
2: 445-446. http://dx.doi.org/10.1136/bmj.2.5759.445
36. Weatherall
DJ. Genetic variation and susceptibility to infection: the red cell and malaria
(2008) Br J Haematol
141: 276-286. https://doi.org/10.1111/j.1365-2141.2008.07085.x
37. Balgir RS. Population and public health implications of child health and
reproductive outcomes among carrier couples of sickle cell disorders in Madhya
Pradesh, India (2014) Intl J MCH AIDS 2: 229-235. https://doi.org/10.21106/ijma.29 38. Balgir RS.
Reproductive outcome in carrier couples of β–thalassemia disorders in a
tertiary hospital in central India (2014) Thalassemia Reports 4: 10-15. https://doi.org/10.4081/thal.2014.1907 39.
Pasvol
G, Weatherall DJ and Wilson RJ. Cellular mechanism for the protective effect of
haemoglobin S against P. falciparum malaria (1978) Nature 274: 701-703. https://doi.org/10.1038/274701a0 40.
Duffy PE and Fried M. Red blood cells
that do and red blood cells that dont: how to resist a persistent parasite
(2006) Trends Parasitol 22: 99-101. https://doi.org/10.1016/j.pt.2006.01.009
41.
Williams TN and Weatherall DJ. World
distribution, population geneticsand health burden of the hemoglobinopathies
(2012) Cold
Spring Harb Perspect Med 2:
a011692. https://doi.org/10.1101/cshperspect.a011692 42.
Chotivanich K, Udomsangpetch R,
Pattanapanyasat K, Chierakul W, Simpson J, et al. Hemoglobin E: A balanced
polymorphism protective against high parasitemias and thus severe P
falciparum malaria (2002) Blood 100: 1172-1176. https://doi.org/10.1182/blood.v100.4.1172.h81602001172_1172_1176 43.
Balgir
RS. Red cell genetic markers in malarial susceptibility and selective advantage
hypothesis (2013) Online Healt Alli Sci 12: 6. 44.
Balgir
RS. Is hemoglobin E gene widely spread in the state of Madhya Pradesh in
central India? Evidence from five typical families (2014) Mediterr J Hematol
Infect Dis 6: e2014060. https://doi.org/10.4084/mjhid.2014.060 45. Genton B, Al-Yaman F, Mgone CS, Alexander N, Paniu MM, et al. Ovalocytosis
and cerebral malaria (1995) Nature 378: 564-565. https://doi.org/10.1038/378564a0 46. Gallagher PG. Red cell membrane disorders (2005) Hematol Am Soc Hematol
Edu Program 1: 13-18. 47. Ruwende
C, Khoo SC, Snow RW, Yates SN, Kwiatkowski D, et al. Natural selection of hemi-
and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria
(1995) Nature 376: 246-249. https://doi.org/10.1038/376246a0
48. Guindo
A, Fairhurst RM, Doumbo OK, Wellems TE and Diallo DA. X-linked G6PD deficiency
protects hemizygous males but not heterozygous females against severe malaria
(2007) PLoS Med 4:
0516-0522. https://doi.org/10.1371/journal.pmed.0040066
49.
Luzzatto L,
Usanga EA and Reddy S. Glucose 6-phosphate dehydrogenase deficient red cells:
resistance to infection by malarial parasites (1969) Science
164: 839-842. http://dx.doi.org/10.1126/science.164.3881.839 50.
Balgir
RS. Contribution of marital distance to community inbreeding, homozygosisand
reproductive wastage for recessively inherited genetic disorders in Madhya
Pradesh, India (2013) Mediterr J Hematol Infect Dis 5: e2013063. https://dx.doi.org/10.4084%2FMJHID.2013.063 51.
Balgir
RS. Impact of consanguinity and inbreeding on homozygosis of recessively
inherited genetic disorders among tribes of central India: The most detrimental
and widely practiced evil (2014) Trib Heal Bull 21: 18-24. Balgir
RS, Department of Biochemistry, National Institute for
Research in Tribal Health (Indian Council of Medical Research), Jabalpur,
Madhya Pradesh, India,
E-mail: balgirrs@yahoo.co.in Balgir RS.Protective resistance by human G6PD enzyme deficiency and hemoglobin
variants against malaria and natural selection: further evidence from review of
new studies (2019)Edelweiss Appli Sci Tech 3: 44-52. G6PD deficiency, Hemoglobin
variants, Thalassemia, Protective resistance, Inverse relationship, Malaria,
Natural selection.Protective Resistance by Human G6PD Enzyme Deficiency and Hemoglobin Variants Against Malaria and Natural Selection: Further Evidence from Review of New Studies
Balgir RS
Abstract
Full-Text
Introduction
Cardinal
Malaria Situation
Human
Red Cell Genetic Variants and Plasmodium Falciparum Malaria
Thalassemia
Sickle
Cell Anemia
Hemoglobin
C Disease
Hemoglobin
E Disease
Cytoskeletal
Abnormalities
Glucose-6-Phosphate
Dehydrogenase Enzyme Deficiency
Red
Cell Genetic Variants and Natural Selection against Malaria
Concluding
Comments
Acknowledgements
References
*Corresponding author
Citation
Keywords