Research Article :
Dylan Amelot, Ammar
Hassoun, Lise-Marie Chamoreau, Hani Amouri and Jamal Moussa Two coordination
polymers of coinage metals with a rare pyridinium-betainoid L assembling ligand
are reported. These polymers are obtained by self-assembly of the linker L and
copper(I) or silver(I) ions in acetonitrile. The compounds were characterized
by spectroscopic methods and by elemental analysis. The solid-state structures
were unambiguously confirmed by single crystal diffraction studies. These
assemblies exhibit original helicoidal arrangements. The UV-Vis. absorption and
photoluminescence properties are reported as well. Metal-Organic Frameworks (MOFs) or
Coordination Polymers (CPs) have attracted a tremendous interest in the past
decade owing to their potential applications as functional materials in various
fields [1-4]. In particular luminescent CPs are attractive candidates for
chemical sensing, light-emitting
devices
or biomedical imaging [5-9]. CPs are also fascinating from a structural point
of view since depending on the nature of the ligand and the metallic bricks, a
huge number of 1-3D frameworks can be obtained such as helicates [10], zeotype
structures [11] for instance. Numerous types of assembling ligands have been designed
and reported in the literature, however we are not aware of any example using pyridinium-betaine like assembling
ligands. We were therefore intrigued to explore the assembling ability of this
family of ligands. It is
noteworthy to mention that betaines possess a very rich optical behaviour,
indeed, these molecules were used as non-linear optical materials [12-15], as
sensors [16-18] and dyes [19-20] for instance. We believe that CPs with betaine
based assembling linkers may provide interesting materials with unusual
properties. In
this work we describe an original approach to the design of CPs using an
unprecedented type of assembling ligand noted (1) that relies on a betaine
scaffold. This ligand consists of a benzimidazolate moiety linked through the C2
carbon atom to the nitrogen of a 4-tert-butylpyridinium core. It has a
zwitterionic electronic structure, a positively charged pyridinium moiety and
an anionic benzimidazolate core that we believed to be capable of coordination
to transition metals through the benzimidazolate nitrogen atoms. We describe in
this work for the first time the preparation and structures of two CPs obtained
by self-assembly of copper(I) and silver(I) ions with this unique assembling
ligand. The copper(I) polymer can be described as a helicate type network in
which the assembling ligand L acts
as the bridge between tetrahedrally coordinated Cu+ cations, while
the silver(I) assembly consists of a honey-comb like 2D network with the ligand
linking two environmentally different Ag+ cations, one silver cation
is linearly coordinated while the neighboring one has a trigonal environment
with three ligands forming a propeller structure around the metal center. The
ligand has been prepared by reaction of 2-chlorobenzimidazole with
4-tert-butylpyridine in refluxing acetonitrile followed by deprotonation of the
NH group in methanol with
cesium carbonate. Compound 1 was isolated as a yellow solid in good yields and
characterized spectroscopically and by elemental analysis. The structure was
clearly identified by 1H-NMR spectroscopy for instance an AABB set
of signals attributable to the benzimidazole protons is observable at δ = 6.95
ppm and δ = 7.48 ppm, finally two doublets due to the pyridinium are visible at
δ = 8.16 ppm and δ = 9.86 ppm. The composition of the target molecule was
determined by mass spectroscopy and by elemental analysis. Mixing
a yellow acetone solution of the ligand
L to a slight excess of [Cu(CH3CN)4]CF3SO3
in acetone (colourless) at room temperature leads to the immediate formation of
a light-yellow precipitate during Four hours. The precipitate was recovered on
a sintered glass funnel and washed with small amounts of ice-cold acetone. The infrared spectrum of this solid is
clearly different than that of the free ligand, it shows in particular presence
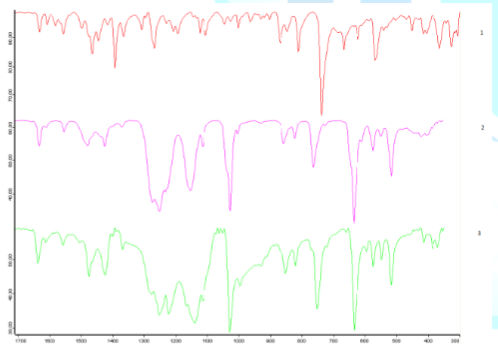
of triflate anions. Figure 1 shows
comparison of infrared spectra of compounds 1, 2 and 3. Figure 1: Impact of coordination of L to copper(I) and silver(I) on its infrared
spectrum. The
solid obtained upon reaction with copper(I) was then dissolved in acetonitrile
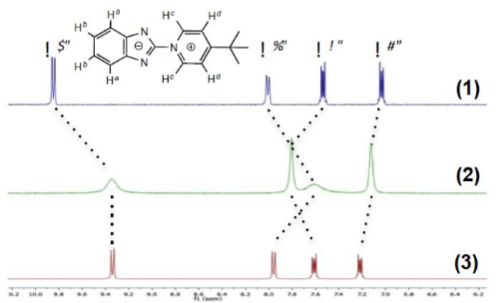
and analysed by 1H-NMR and 13C-NMR spectroscopy. The 1H-NMR spectrum displays
important changes with regard to the free ligand (Figure 2) but the symmetry pattern of the free ligand is
maintained. First one can note the important broadening of the aromatic signal
due to coordination to copper(I), it is well known that copper(I) causes
broadening of 1H-NMR signals due to its quadrupolar nucleus [21]. This
broadening is certainly also amplified by a dynamic exchange with the
coordinating acetonitrile solvent. The chemical shifts of the pyridinium
protons are the most affected upon coordination. This observation confirms that
coordination of the ligand to copper(I) occurs in solution even in acetonitrile
which is a coordinating solvent, however a dynamic equilibrium cannot be
excluded as it will be shown with the absorption and emission data in this
solvent. Solution
1H-NMR study of the silver(I) solid shows a similar behaviour.
Although without significant broadening of the signals, both the benzimidazole
moiety and the pyridinium protons undergo important shifts presumably due to
coordination to the cations. Compound {[AgL]CF3SO3}n (3) exhibited lower solubility in
acetonitrile compared to the copper compound {[CuL(CH3CN)2]CF3SO3}n
(2). These
solids were then dissolved in acetonitrile and crystallized by slow diffusion
of diethyl ether into these solutions. Single-crystals suitable for an X-Ray diffraction structure
determination
were obtained to unravel the structural features of the novel compounds. In
both cases the analysis shows formation of coordination polymers with the
assembling ligand bridging copper(I) or silver(I) ions providing compounds of
general formulae {[CuL(CH3CN)2]CF3SO3}n
(2) and {[AgL]CF3SO3}n (3) respectively. The structural
features are discussed in the next section. The
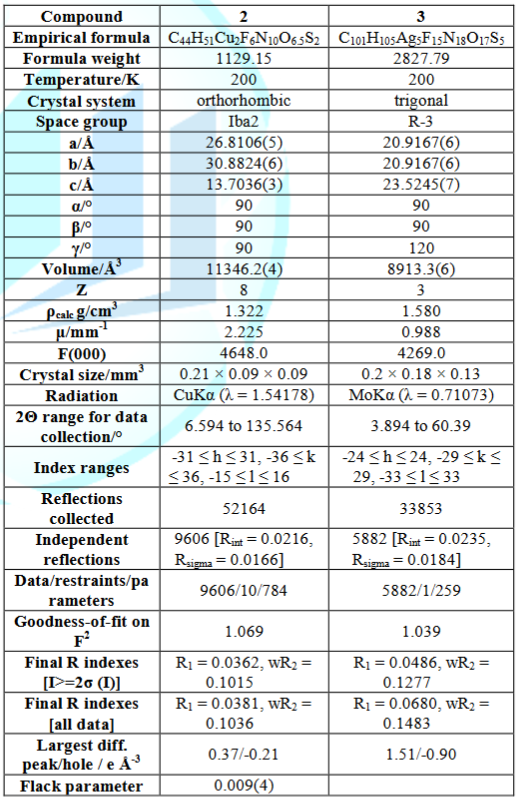
copper(I) compound crystalizes in the orthorhombic space group lba2, with
unit-cell dimensions a = 26.8106(5), b = 30.8824(6), c = 13.7036(3) Å. The
structure clearly shows that the assembling ligand is bridging two tetrahedral
coordinated copper(I) ions through each nitrogen of the anionic benzimidazolate
ring providing a 1D helicoidal structure. Every copper(I)
center is therefore linked to two benzimidazolate nitrogen of the benzimidazolate
cores with two coordinated acetonitrile molecules completing the coordination
sphere. The copper atoms are in a slightly distorted tetrahedral environment.
The two aromatic parts of the coordinated ligand are not coplanar but twisted
by approximately 45°C. Cu-N bond distances lie in the range of reported values
for related compounds in the literature. Thus the coordination polymer obtained
with copper(I) ions can be formulated as {[CuL(CH3CN)2]CF3SO3}n
(2). The asymmetric unit consists of subunits containing two copper(I) atoms,
two ligands and four coordinated acetonitrile molecules. These views clearly
show the helix feature of this structure that is grown along the c axis of the
unit cell. This asymmetric unit is repeated to build the polymeric chains, the
helical repeat distance is twice the c axis translation. This compound is
obtained as a racemic mixture no spontaneous
resolution was observed during crystallization. Reaction
using silver(I) triflate instead of copper(I) ions provided upon
crystallization from acetonitrile suitable single-crystals for an X-Ray diffraction analysis. The obtained silver(I)
coordination polymer can be formulated as {[AgL]CF3SO3}n
(3) and crystallizes in the R-3 space group. The structure shows that this silver(I)
assembly consists of a honey-comb like 2D network with ligand bridging two
environmentally different siver(I) cations, one silver cation is linearly
coordinated while the neighbouring one has a trigonal environment with three ligands
forming a propeller structure around the metal centre. These
two alternating coordination environments lead to formation of six subunits
membered cyclic subunits that are repeated to describe a honeycomb type 2D
network. The Tricoordinated silver cations exhibit a chiral P or M propeller
structure, the arrangement is homochiral in one plane leading to a racemic
crystal i.e. no spontaneous resolution occurs in the crystallization process. These
results show that the benzimidazolato moiety of the novel
ligand is nucleophilic enough to coordinate transition metal cations despite
presence of the neighbouring cationic pyridinium ring. Consequently we can
reasonably believe to obtain complexes in which this (pro)-ligand could behave
as a chelating C^N upon C-H bond activation (deprotonation)
in position as to the pyridinium nitrogen. The
resulting carbon centre will be a pyridylidene that might impact the electronic
properties of the target complexes. In particular, the desired metal complexes
could exhibit interesting luminescent properties giving the luminescence
ability of ligand. The absorption spectra of the ligand
and the metal assemblies were recorded in acetonitrile at C = 10-5 m-1
and are assembled in Figure 4. Table 1: Crystallographic
parameters for 2 and 3. The
spectrum of the free ligand shows a high-energy absorption band centered at 273
nm with high molar absorptivity (> 80000 m-1.cm-1)
indicating a π-π* process, the spectrum also exhibits a lower energy transition
centered at 395 nm ascribed to an intense charge transfer absorption in line
with observed values in related betainoid compounds reported in the literature
[22]. Absorption
and photoluminescence properties The
metal assemblies show spectra with a similar pattern to the free ligand showing
weakly metal perturbed transitions. This behavior indicates a fast dynamic
equilibrium involving free and coordinated ligand L competing with the
acetonitrile solvent molecules (Figures
5,6). Acetonitrile solution (10-5
M) of the free ligand shows a strong emission at lem around 620 nm upon excitation in the
charge transfer absorption region. This emission is ascribed to a CT process
according to studies on related molecules [22].
The metal assemblies both still exhibit this emission almost not
affected by the coordination maybe due to fast dynamic equilibrium in the
coordinating acetonitrile solvent as suggested by the absorption behavior as
well. These
preliminary photoluminescence experiments show that luminescence can be
maintained in metal assemblies with this type of ligands, which is promising
for the development of luminescent metal complexes with various
potential applications. All
experimental manipulations were carried out under an argon atmosphere by using Schlenk tube techniques. Solvents were
dried and distilled under argon by standard procedures. All reagents obtained
from commercial sources were used as received. The 1H and 13C
NMR spectra were recorded in CD3CN using a Bruker Avance 300 NMR
spectrometer at 300.13 MHz, and 75.47 MHz respectively. IR spectra were
recorded on a Bruker Tensor 27 equipped with an ATR Harricks apparatus. UV-Vis.
spectra were recorded on a JASCO V-670 Spectrometer. Photoluminescence spectra
were recorded using a JASCO J-815 CD Spectrometer. A
single crystal of each compound was selected, mounted onto a cryoloop, and
transferred in a cold nitrogen gas stream. Intensity data were collected with a
BRUKER Kappa-APEXII diffractometer with either graphite-monochromated Mo-Kα or
copper micro-focused radiation. Data collection was performed with APEX2 suite
(BRUKER). Unit-cell parameters refinement, integration and data reduction were
carried out with SAINT program (BRUKER). SADABS (BRUKER) was used for scaling
and multi-scan absorption corrections. In the Olex2 [23], the structure were
solved with SHELXT-14 [24] program and refined by full-matrix least-squares
methods using SHELXL-14 [25]. 2-chlorobenzimidazole
(540 mg, 3,54 mmol) and anhydrous 4-tert-butylpyridyl (1 ml) were placed in a
Schlenk tube and acetonitrile (20 ml) was added. The mixture was refluxed
overnight under an argon atmosphere at 100°C. Then cooling the solution
resulted in the precipitation of unreacted 2-chlorobenzimidazole as white
crystalline needles that can be recovered on a glass frit. The filtrate is then
evaporated to dryness and to the oily residue is added anhydrous diethyl ether
(50 ml), this precipitates and anhydrous Cs2CO3 (219 mg)
were placed in a Schlenk tube and methanol (20 ml) was added. The mixture was
stirred under an atmosphere of argon at room temperature for two hours. The
solvent is then evaporated under reduced pressure. The residue is extracted with
dichloromethane (50 ml), compound 1 is obtained as a yellow crystalline solid
(553 mg, 1,92 mmol). Yield: 54 %. 1H NMR (300.13
MHz, DMSO): δ = 9.84 (d, 2H, 2J = 6.9 Hz, Hα pyridynium),
8.01 (d, 2H, 2J = 6.9 Hz, Hβ pyridynium), 7.53 (dd, 2H, 2J
= 9.0 Hz, 3JH-H = 2.7 Hz, Hβ benzimidazol),
7.03 (dd, 2H, 2J = 9.0 Hz, 3JH-H = 2.7 Hz, Hα
benzimidazol), 1.46 (s, 9H, CH3 pyridynium). 13C{-1H}
NMR (75.47 MHz, DMSO) δ=29.1, 36.2, 100.3, 119.8, 124.3, 138.3, 146.7, 154.3. HRMS
[L- K]+ calc. 252.1495, Found. 252.1505 A
colorless solution of [Cu(CH3CN)4]CF3SO3
(100 mg, 0.40 mmol) was added to a yellow acetone solution of the ligand (150
mg, 0.39 mmol) at room temperature during four hours. Immediate formation of a
light-yellow precipitate was observed. The precipitate was separated on a sintered glass funnel and washed with
small amounts of ice-cold acetone. The precipitate was dissolved in the minimum
of acetonitrile (ca 4-5ml) and slow diffusion of diethylether into this
solution provided light yellow crystals of compound 2, separated by filtration
on a sintered glass funnel and dried under vacuum overnight (184 mg, 0,34
mmol). Yield: 86%. anal. Calcd (%) for C17H17CuF3N3O3S
(463, 9 g.mol-1): C 44.01, H 3.69, N 9.06, found: C 43.42, H 3.73, N
8.81. (Precipitate before crystallization in acetonitrile) 1H NMR (300.13 MHz,
CD3CN): δ = 9.35 (br, 2H, Hα pyridynium), 7.81 (m br, 2H, Hβ
benzimidazol), 7.61 (m br, 2H, Hβ benzimidazole Hβ
pyridynium), 7.12 (m br, 2H, Hα benzimidazol), 1.36 (s, 9H, CH3
pyridynium). 13C{-1H} NMR (75.47 MHz, CD3CN) δ
= 29.0, 36.4, 121.1, 122.8, 124.0, 148.4, 171.8, 191.8. This
compound was prepared in a similar way to compound 2 mixing AgCF3SO3
(145 mg, 0.56 mmol) instead of [Cu(CH3CN)4]CF3SO3
with ligand (145 mg, 0.58 mmol). The obtained precipitate was
recrystallized from acetonitrile/ diethylether (271 mg, 0.54 mmol). Yield: 95
%. Anal.
calcd. (%) for C17H17AgF3N3O3S
(508,3 g.mol-1): C 40.17, H 3.37, N 8.27, found: C 42.89, H 3.86, N
8.48. (Precipitate before crystallization in acetonitrile). 1H NMR (300.13
MHz, CD3CN): δ = 9.38 (d, 2H, 2J = 6.9 Hz, Hα
pyridynium), 7.88 (d, 2H, 2J = 6.9 Hz, Hβ pyridynium),
7.58 (dd, 2H, 2J = 9.0 Hz, 3JH-H = 2.7 Hz, Hβ
benzimidazol), 7.16 (dd, 2H, 2J = 9.0 Hz, 3JH-H =
2.7 Hz, Hα benzimidazol), 1.40 (s, 9H, CH3 pyridynium). 13C{-1H}
NMR (75.47 MHz, CD3CN) δ = 29.0, 36.6, 122.0, 124.7, 140.9, 144.03
(complete data could not be obtained due to precipitation occurring during the
spectrum record). In summary we have
reported two coordination polymers with a rare pyridinium-betaine based assembling
ligand and coinage metals. The self-assembly of this rare ligand with copper(I)
or silver ions provides appealing coordination networks with helicoidal structures.
Our future objectives will focus on the C-H bond activation in the α position
to the pyridinium nitrogen to generate bidentate
pyridylidene chelates which is a still a challenging task. 1.
Rosi N L, Eckert J, Eddaoudi M,
Vodak D T, Kim J, et al. Hydrogen storage in microporous metal-organic
frameworks (2003) Science 300: 1127-1129. 2.
Eddaoudi M, Kim J, Rosi N,
Vodak D, Wachter J, et al. Systematic design of pore size and functionality in
isoreticular mofs and their application in methane storage (2002) Science 295:
469-472. 3.
Kitagawa S and Uemura K.
Dynamic porous properties of coordination polymers inspired by hydrogen bonds
(2005) Chem Soc Rev 34: 109-119. https://doi.org/10.1039/b313997m
4.
Kitagawa S, Kitaura R and Noro
SI. Functional porous coordination
polymers (2004) Angew Chem Int Ed 43: 2334-2375. https://doi.org/10.1002/anie.200300610 5.
Batten and Stuart R.
Coordination Polymers: Design, Analysis and Application (2008) RSC Publishing
pp: 297-307, 396-407. https://doi.org/10.1002/anie.200902588
6.
Tong ML, Hu S, Wang J, Kitagawa
S and NgS W. Supramolecular isomerism in cadmium hydroxides phases (2005)
Crystal Growth & Design 5: 837-839. 7.
Yuming Y, Qiang Z, Wei F and
Fuyou L. Luminescent chemodosimeters for bioimaging (2013) Chem Rev 113:
192-270. 8.
Moussa J, Boubekeur K and Amouri
H. Self-Assembly of 1-D coordination polymers using organometallic linkersand
exhibiting argentophilic interactions AgI···AgI (2005)
Eur J Inorg 19: 3808-3810. https://doi.org/10.1002/ejic.200500605
9.
Moussa J, Guyard-Duhayon C,
Boubekeur K, Amouri H, Yip S K, et al. Self-Assembly of one and two-dimensional
coordination polymers with quinonoid backbones featuring coinage metals as
nodes (2007) Cryst Growth & Design 7: 962-965. 10. Sujit KG and Parimal KB. Self-Assembly of lanthanide helicate
coordination polymers into 3d metal-organic framework structures (2004) Inorg
Chem 43: 2293-2298. https://doi.org/10.1021/ic034982v
11. Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, et al.
Metal-organic framework materials as catalysts (2009) Chem Soc Rev 38 :
1450-1459. https://doi.org/10.1039/b807080f 12. Perrier A, Aloïse S, Pawlowska Z, Sliwa M, Maurel F, et al.
Photoinduced intramolecular charge transfer process of betaine pyridinium: a
theoretical spectroscopic study (2011) Chem Phys Lett 515 : 42-48. https://doi.org/10.1016/j.cplett.2011.09.013
13.
Kharlanov V and Rettig JW.
Experimental and theoretical study of excited-state structure and relaxation
processes of betaine-30 and of pyridinium model compounds (2009) Phys Chem A
113: 10693-10703. 14.
Mente RS and Maroncelli M.
Computer simulations of the solvatochromism of betaine-30 (1999) J Phys Chem B
103: 7704-7719. https://doi.org/10.1021/jp991549r
15. Reichardt C. Solvatochromic Dyes as solvent polarity indicators
(1994) Chem Rev 94: 2319-2358. 16.
Reichardt C. Solvents and
solvent effects in organic chemistry (1988) Weinheim 148: 534. https://doi.org/10.1002/anie.201105531
17.
Abe J and Shirai Y.
Heterocyclic betaines exhibiting extremely large first hyperpolarizability: ab
initio and indo/s calculations (1996) J Am Chem Soc 118: 4705-4706. 18.
Abe J, Shirai Y, Nemoto N,
Miyata F and Nagase Y. Heterocyclic Pyridinium Betaines, A new class of
second-order nonlinear optical materials: combined theoretical and
experimental investigation of first-order hyperpolarizability through ab
initio, indo/s, and hyper-rayleigh scattering (1997) J Phys Chem B 101:
576-582. http://dx.doi.org/10.1021/jp961711f
19.
Tiexin Z, Xiangyang G, Yusheng
S, Cheng H and Chunying D. Dye-incorporated coordination polymers for direct
photocatalytic trifluoromethylation of aromatics at metabolically susceptible
positions (2018) Nature Communications 9: 1-6. 20.
Wen T, Zhang XD and Zhang J.
Two-Dimensional Copper(I) Coordination Polymer Materials as Photocatalysts for
the Degradation of Organic Dyes (2013) Inorg Chem 52: 12-14. https://doi.org/10.1021/ic302273h 21.
Abragam A. The Principles of
Magnetic Resonance (1961) Oxford University Press, London. 22.
Aloïse S, Pawlowska Z, Poizat
O, Buntinx G, Perrier A, et al. Excited-state dynamics of thiophene substituted
betaine pyridinium compounds (2014) Phys Chem Chem Phys 16: 1460-1468. https://doi.org/10.1039/C3CP53614A
23.
Dolomanov
OV, Bourhis LJ, Gildea RJ, Howard JAK and Puschmann H. OLEX2: A complete
structure solution, refinement and Analysis program (2009) J Appl Cryst
42: 339-341. https://doi.org/10.1107/S0021889808042726 24.
Sheldric
GM. SHELXT-Integrated space-group and crystal-structure determination (2015) Acta Crystallographica Section A 71: 3-8. https://doi.org/10.1107/S2053273314026370 25.
Sheldric
GM. Crystal structure refinement with SHELXL (2015) Acta
Crystallographica Section C 71:
3-8. https://dx.doi.org/10.1107/S2053229614024218 Moussa
J, Sorbonne University, Paris Institute of Molecular
Chemistry, Paris, France, Tel: (33)1-44-27-60-90, E-mail: jamal.moussa@sorbonne-universite.fr
Appealing Copper(I) and Silver(I) Coordination Polymers with an Unprecedented Betainoid Assembling Ligand
Abstract
Full-Text
Introduction
Results
and Discussion
Synthesis
and Characterization
Structural
determination and analysis
![Views of the cationic part of the coordination polymer {[CuL(CH3CN)2]CF3SO3}n (2). Hydrogen atoms and triflate anions are omitted for clarity.](http://edelweisspublications.com/edelweiss/figures/ecs-18-107_figure_3.png)
![View of the cationic part of the coordination polymer {[AgL]CF3SO3}n (3). Hydrogen atoms and triflate anions are omitted for clarity.](http://edelweisspublications.com/edelweiss/figures/ecs-18-107_figure_4.png)
![UV-Vis. Absorption spectra of the free ligand L and coordination polymers {[CuL(CH3CN)2]CF3SO3}n (2) and {[AgL]CF3SO3}n (3).](http://edelweisspublications.com/edelweiss/figures/ecs-18-107_figure_5.png)
![Photoluminescence spectra of the free ligand l and coordination polymers {[cul(ch3cn)2]cf3so3}n (2) and {[agl]cf3so3}n (3). (exc =](http://edelweisspublications.com/edelweiss/figures/ecs-18-107_figure_6.png)
Experimental
Compound
1
Compound
2
Compound
3
Conclusion
References
*Corresponding author