Research Article :
Static magnetic field (0.5 T) effects on water
evaporation rate from anionic Sodium Dodecyl Sulfate (SDS) and cationic Dodecyl
Trimethyl ammonium Bromide (DoTAB) 1 mM solutions were studied at room
temperature and humidity for up to several hours. Keeping in mind possible
practical application of the effects the experiments were intentionally carried
out in a common laboratory environment and not in any sophisticated conditions.
The evaporation of water from Magnetic Field (MF) treated and untreated samples
were carried out simultaneously in the same environment. Although the
quantitative differences in the evaporated amounts of water between MF treated
and untreated samples changed from run to run, the qualitative MF effects were
always reproducible. Therefore, it is believed that the observed changes are
significant. It was found that the MF affects evaporation rate of water from
solutions of both surfactants causing increase in the evaporated water amount
in comparison to that of MF untreated sample. Prior to MF experiments first the
water evaporation rate from the untreated surfactants solutions was studied.
From the MF-untreated anionic surfactant solution water evaporated slower than
from pure water, while from the cationic one water evaporated faster than from
pure water. This difference was explained taking into account the properties of
the polar (ionic) head of the surfactants, i.e. their size, ability to hydrogen
bonding formation with water molecules, and the reduction of water surface
tension. The MF treatment caused an increase in the evaporated water amount
from both surfactants. However, a greater effect was observed for cationic
DoTAB. Because the hydrocarbon tail in both surfactants is the same (C12)
the observed differences were assigned to the differences in their ionic heads.
Gibbs adsorption equation and Lorentz force in the gradient MF were applied to
explain the differences. Introduction Investigation of Magnetic
Field (MF) effects on properties of water and
aqueous solutions are still of interest although they have been studied for at
least 50 years. Hundreds of papers have been published where magnetic fields
effects and application of MF in industry, agriculture, medicine, and others
are described. Nevertheless, some of the results are debatable or even
incompatible. Initially MF studies were focused to eliminate the hard scale
formation at elevated temperatures in industrial pipes or house heating
installations. If MF would successfully protect against deposition of the
carbonates this could be beneficial elimination of chemicals used for water
softening which are expensive and harmful for the environment. Later studies of
MF effects in many systems and applications were carried out. Generally, using
the classical magnetic field theory it is hardly to explain the observed
effects which, however, often are well documented and statistically validated.
The latest theories claim that to obtain an MF effect more important is the
field gradient than its strength [1-3]. Also, the non-classical theory of
nucleation mechanism and formation of dynamically ordered, so called liquid
like oxyanion polymers, are used to explain the magnetic field action [4,5]. For this purpose static MF (max.
0.5 T) originating from ring Nd magnets (MP 86 x 58 x 35 mm) was applied in
which the sample of 10-3 M anionic Sodium Dodecyl Sulfate (SDS) or
cationic Dodecyl
Trimethylamonium Bromide (DoTAB)
solutions were placed in the magnet for up to 2-3h in an open plastic vessel.
Simultaneously, another vessel as a reference with the same surfactant solution
was placed ca.1.5 m apart from that with the MF and the samples were separated
by a wooden board. All the experiments were carried out at room temperature and
humidity. Every 30 min the vessels were weighed with the accuracy 0.1 mg, and
the evaporated amount of water was calculated. For the reference purposes
evaporation of water from the surfactant solution and pure Milli-Q water
without MF presence were investigated too. In the previous paper similar
studies have been conducted on MF effects on evaporation rate of pure water and
water surface tension [21]. These are preliminary studies and depending on the
obtained results further systematic investigations in aspect of possible
practical applications will be continued. Application of MF for enhancing or
hindering water evaporation, depending on the need of the process, would be
beneficial. Materials Sodium dodecyl sulfate >99.0%
was purchased from Fluka and dodecyl
trimethylammonium bromide approx. 99%
was from Sigma. Both were used without further purification. For preparation of
their 10-3M aqueous solutions water from Milli-Q Plus system was
used and the solutions were prepared a day before their first usage. Methods For the experiments of evaporation
rate 65 mL (in some cases 50 mL, see Figure 8) samples of the surfactant
solution or water from Milli-Q Plus system (resistivity 18.2 MΩcm) were used.
The magnetic field originated from a neodymium ring magnet 86 mm (outer
diameter) x 58 (inner dimeter) x 35 mm (height) directed with its north N
or south S pole upward. The solution or water surface in
the vessel was exactly on the level of the magnet edge and the vessel outer
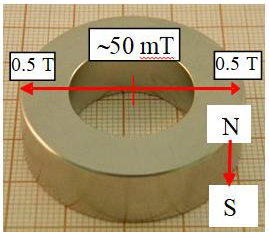
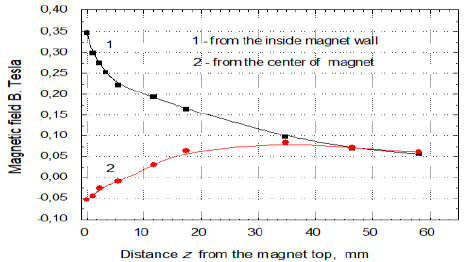
diameter was 53.6 mm while the inner one was 52 mm. Figure 1a shows the magnet used and Figures 1b and 1c present changes of the magnetic field strength
perpendicularly from the solution surface at the inner edge and at the center
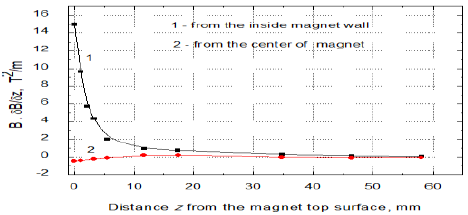
of magnet. In Figure 1c the changes of MF gradients at the same places as in
Figure 1b are plotted respectively. Figure 1a:
The used magnet and the magnetic field strength change across the radial
distribution. In Figure
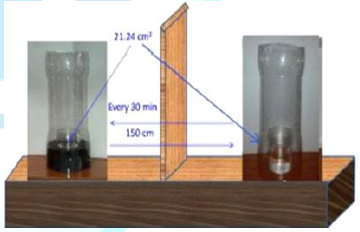
2 the setup for the evaporation experiments is shown. The same setup and
procedure were used in the study of MF effects on pure water published earlier
[21]. As it is seen in Figure 2 during the evaporation experiments the
MF-treated and MF-untreated samples evaporated simultaneously at the room
temperature (23 ± 1°C) and the relative
humidity was 32-38%. The surface area of the sample on which the MF acted was
21.24 cm2 and the circular surface radius was 26 mm. Therefore the
meniscus curvature effect on the vapor pressure (Kelvin equation) did not play
any role at this sample diameter. From Figures 1b and 1c it is seen that the strongest MF effect
in vertical direction appears at the sample vessel walls and it vanishes toward
the sample center. In the surface radial direction it decreases from 0.35T to
0.05T. After every 30 min the closed samples were weighed and then their
location together with the magnet was replaced. To avoid any possible influence
of air fluctuations in the room the samples were placed in plastic tubes (Figure
2). The samples were weighed using a high precision balance Sartorius with an
accuracy of 0.1 mg. The amounts of evaporated water were calculated by subtracting from the initial weight of the
MF-treated or MF-untreated sample its weight after given time of the
evaporating experiment, respectively. Then the difference between these two
amounts was calculated and plotted versus time. Figure 2: Setup for the solution evaporation experiments. First
to learn whether water evaporation rate is different from aqueous surfactant
solutions than that from pure water experiments without MF were performed using
10-3M SDS solution and pure water. In Figure
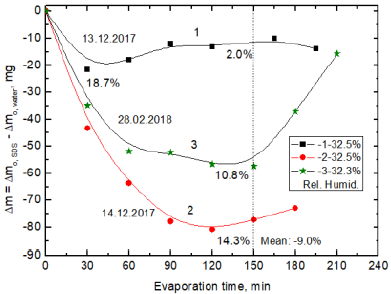
3 are shown the results where it can be seen that more water evaporates
from pure water than from 10-3M SDS solution (the negative differences in mg).
However, the differences obtained in these three separate experiments differ
between themselves. The room temperature was the same 22oC and the relative
humidity did not differ much. The plateau on the curve indicates that the rate
of water evaporation is the same from MF treated and untreated samples and an
extremum show the greatest difference in the evaporation rate. The difference
in the evaporated amounts can be also presented as the relative percentage
taking the evaporated amount from the pure water (or MF-untreated sample) as
the reference 100%. In Figure 3 are shown these relative negative percentages
calculated after 150 min of the experiment duration. Thus, the reduced
evaporation of water from the SDS solution amounts to 2.0%, 10.8% and 14.3%,
respectively which gives mean reduction percentage 9%. In experiment 1 (Figure
3, curve 1) the 2.0% relative decrease after 150 min results from 511.2 mg and
521.4 mg evaporated water from the SDS solution and pure water. However, after
30 min of the MF treatment in this experiment the percentage reduction amounted
to 18.7% (Figure 3). It is because during this time 94.1 mg and 115.8 mg of
water evaporated from the SDS solution and pure water, respectively. Therefore
to better depict the changes in the next figures, presenting the differences in
amounts of evaporated water in mg, also the relative percentage values are
given for 150 min of the experiment duration. As can be seen in Figure 3 in
next two experiments much larger difference in the evaporated amounts of water
has been obtained. Hence, the mean relative smaller amount of the evaporated
water from the SDS solution after 2h is 9.0%. These results show that even
without MF presence it is difficult to reproduce exactly the evaporation rate
of water in a typical room environment using the same experimental setup, the
surfactant lot and water. Nevertheless, an important finding is that water
evaporates faster from pure water than 10-3 M SDS solution. However, one would
expect an opposite relationship, i.e. faster and enhanced evaporation of water
from the SDS solution whose surface tension is lower than pure water, 49.01 ± 0.26 mN/m (10-3 M SDS) and 72.30 ± 0.22 mN/m, respectively. This
indicates that the cohesion forces between water molecules are stronger on the
surface in pure water (2 × 72.8=145.6 mN/m) than in the SDS solution. Despite
that 10-3 M SDS solution concentration is less than its critical micelle
concentration, CMC=8.2 ×10-3 M at 25°C, there is already significant amount of
SDS molecules adsorbed on this solution surface (compare the surface tensions).
The molecules are oriented with their hydrocarbon chains toward the air and the
ionic heads are located between the water molecules. The -SO4- head of SDS
posseses oxygen atoms which interact with water molecules by hydrogen bonds.
They are much stonger than the London dispersion and Keesom dipole-dipole
forces, i.e. the strength of hydrogen bonds in water is ca. 20 kJ/mol while
that of London and Keesom is 0.4-4 kJ/mol [22]. Van Oss and Constanzo [23]
reported surface tension for SDS molecules immersed in water to be 23.8 mN/m
for the hydrocarbon tail and 34.6 mN/m for the electron-donor parameter due to the presence of -SO4- head
responsible for the hydrogen bonds formation. The average distance between the -SO4-
groups in water was evaluated to be 0.907 nm. While between two alkyl chains
the attraction amounts to102.1 mJ/m2 so strong repulsion of the electrostatic
and polar nature exists between the sulfate heads. Therefore, they have to
diverge with at least one -CH2- to which -SO4- is attached. From the surface
tension values of SDS solution cited above it results that the interactions are
responsible for SDS surface tension to a great
degree. In
the next series of experiments the effect of static MF on the evaporation rate
of water from 10-3 M solution of SDS was studied using the setup
shown in Figure 2. The obtained results of five individual experiments
performed during different timespan are plotted in Figure 4. In the all cases, except for one, more water evaporated
from magnetized
solutions.
In one experiment during first 2h water evaporated faster from MF-untreated
solution. However, at a longer MF treatment time the evaporation rate from
magnetized solution significantly increased and after next 2h the difference in
the evaporated amount of water from MF-treated sample was similar to the two others
MF-treated samples (Figure 4, curves 3 and 5). Interestingly no clear
relationship between the MF direction (north N or south S pole directed upward)
and the evaporation rate has been observed. Hypothesizing similarly as above
for the MF-untreated SDS samples, the observed faster evaporation of water from
the MF-treated samples would result from a smaller number of SDS molecules
adsorbed on the surface after the treatment. However, it is probably not the
case because the surface tension of MF treated10-3M solution
decreases by ca. 4 mN (to be published in the Part II of this paper) and hence
according to the Gibbs adsorption equation it means that the surface excess
concentration has increased. Where: is
the surface excess concentration of component 2 (surfactant) relative to its concentration in
the bulk solution at zero excess concentration of main component 1(water). Therefore,
the reason of increased water evaporation from MF treated solution might be due
to weakening of Van der Waals
interactions
and hydrogen bonds in water intra-clusters [24,25] and formation of hydrogen
bonds of water with the oxygen atoms from -SO3- groups. The average
increase in the evaporated water after 150 min MF treatment amounts to 4.5% but
it changes between 1.0% and 8.5% depending on the experiment run (Figure 4). To
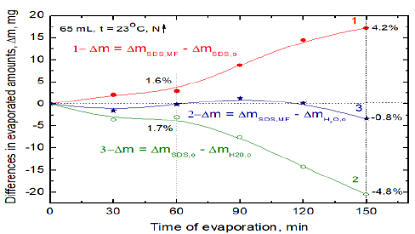
better depict the MF effects and compare water evaporation from the SDS
solution and from pure water the differences are plotted in Figure 5 where curve 1 is that 1 N from
Figure 4 (these values are about the mean ones) and the curves 2 and 3 were
calculated using the values obtained for pure water on the same day as those of
curve 1. As can be seen in Figure 5 during 1h less water evaporated from the
MF-untreated 10-3 M SDS solution than from pure water (1.7%, curve
2). From the MF-treated SDS solution more water evaporated from this solution
than the MF-untreated one (1.6 %, curve 1). After timespan 150 min the
differences increased to 4.8% (curves 2) and 4.2% (curves 1), respectively.
However, within 2h the evaporation rates of water from MF-treated SDS and pure
water appeared to be practically the same and decreased by 0.8% only after 150
min (curve 3). Curves:
1- between magnetized and non-magnetized solutions; 2-between non-magnetized
solution and pure water; 3-between magnetized solution and pure water. The
relative differences in the evaporated amounts are given in percentage. Analogous
experiments to those with the anionic SDS surfactant were carried out with 10-3M
cationic dodecyltrimethyl ammonium bromide, DoTAB, solution. Similarly as in
the case of SDS solution first it was interesting to compare water evaporation
rate from 10-3 M DoTAB aqueous solution and pure water without MF
presence. Two series of experiments have been carried out and the results are
plotted in Figure 6. While from the
anionic SDS solution water evaporated faster from pure water samples, so in the
case of cationic DoTAB solution water evaporated faster from its solution than
from pure water. In other words, at the same experiment duration more water
evaporated from MF-untreated DoTAB sample than pure water, hence the
differences in Figure 6 are positive. Also the percentage changes of evaporated
water amounts are calculated for 150 min experiment duration and also at the
end of particular experiment. The mean value from 7 experiments amounts 5.1% of
the increased amount of evaporated water from DoTAB while in the case of SDS
solution 9% less of water evaporated from the solution than from pure water
(Figure 3). In the room environment at a slightly changing humidity and
temperature obtained differences vary from run to run of the experiment but no
doubt each time the evaporated amount of water from the solution is larger than
from pure water. Next
the DoTAB sample was MF treated during water evaporation and simultaneously
water from a reference untreated sample evaporated too (Figure 2). The results
of differences in the amounts of evaporated water from MF treated
and untreated solutions are presented in Figure
7 for four individual experiments. It can be seen in the figure that the MF
causes increase in the evaporated amount of water in comparison to the
MF-untreated solution. The relative percentage from 150 min experiment duration
lies between 1.9-9.3%, giving mean value 4.6% which is practically the same as
the mean value for SDS MF-treated solutions (4.5%, Figure 4). Similarly as in
the case of SDS solution the MF field direction (north or south pole upward)
does not make any visible difference. Also it looks that the small changes in
relative humidity (32% and 36%) do not influence significantly the evaporation. The surface
tension of 10-3 M DoTAB solution after 60 min MF treatment decreased
by 11.4 mN/m from 60.7 mN/m to 49.3 mN/m, i.e. more than in SDS solution, by
5.7 mN/m (to be published in the Part II of the paper). Taking again into
account the Gibbs adsorption equation (Equation 1) for binary solutions a
decreasing surface tension with the increasing bulk concentration (activity) of
a surfactant means an increase in the surface excess concentration of this
surfactant G2(1). Therefore, it
can be concluded that MF causes an increased adsorption of these two surfactant
molecules on the surface, especially that of cationic DoTAB. In other words, it
can be concluded that MF affects the structure of surfactants surface layer in
a similar way as it occurs during the increasing bulk concentration of
surfactant. Then,
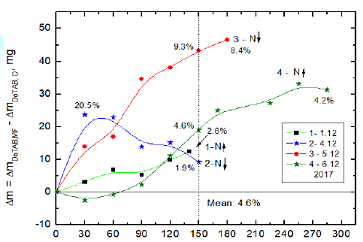
an evaporation experiment was carried out using simultaneously three samples,
i.e. MF-treated DoTAB sample, another MF-untreated sample and pure water. Thus
obtained results are plotted in Figure 8.
Additionally, for comparison curve 1b shows the biggest differences obtained
after MF treatment (curve 3 from Figure 7). The 150-min MF treatment enhances
water evaporation from the DoTBA solution between 2.6 % (curve 1a) and 9.3% in
the extreme case (curve 1b). This extreme effect is comparable with the
differences between evaporating water from the MF-untreated DoTAB solution and
pure water (curve 2). In comparison to pure water the 150 min MF treatment
enhances evaporation of water by 12.5% (curve 3). Comparing these results with
those for SDS solutions (Figure 5) much stronger MF effect is clearly seen in
the case of cationic
surfactant. Figure 8: Differences
between evaporated water amounts from MF-treated and MF-untreated DoTAB
solutions and pure water. Comparison
of the MF effects on the surfactant solutions To
compare the observed MF effects on the two kinds of surfactants, first the
differences in their ionic heads should be discussed. This is because both
surfactants have the same hydrocarbon chain length, C12, therefore
the different behaviour can be ascribed to drastic differences in the head
group properties. They possess completely different ionic heads, i.e. -OSO3-Na+ and
-N+(CH3)3Br–: The surface activity
of surfactants can be described by Sprow and Prausnitz equation [20]. For Equation 2 refer PDF In
Equations 2 and 3 the activity a of
the components are defined in symmetrical system, i.e. aw, as
®1 if xw,
xs ® 1. On the basis of the above equations
Zdziennicka et al. [28] found for many surfactant solutions the maximum
reduction of water surface tension to be ca. 41 mN/m, what indicated that the
chains are oriented parallel toward the surface. This value is smaller than the
values of our SDS and DoTAB solutions measured after MF treatment. The bigger
decrease in surface tension of the cationic surfactant than the anionic after
MF treatment can be ascribed to the presence of three -CH3 groups in
the DoTAB head group whose surface tension is lower than -CH2- group
present in the hydrocarbon tail [29,30]. Hence the decrease in surface tension
after MF treatment can be due to the molecules reorientation. More detailed
discussion will be given in the paper to follow (in Part II) on the MF effect
on the surface tension of these two surfactants. Basing on the above results it
can be concluded that the magnetic field causes changes in the structure of the
surface layer of adsorbed surfactant molecules and because of different surface
properties of -N+(CH3)3 and -O-SO3–
groups the former causes water evaporation easier while the later harder. It
is important to recognize possible mechanisms of the MF force action. Some
approaches were discussed in the previous paper dealing with water evaporation
from pure water
surface
[21]. Nakagawa et al. [13] and others [2-5,18] found that for water evaporation
in the MF field more important is the field gradient B×dB/dx than the field
itself. Moreover, oxygen present in the air can cause a susceptibility gradient
in the direction normal to evaporating water surface which can enhance magnetic
convection and in consequence a decrease in the water vapor density. This is because
volume susceptibility c of oxygen is
much greater than that of water and nitrogen. The bulk magnetic force was
calculated by the authors [13] from Equation (4). For Equation 4 refer PDF In
the case of electrolyte solution first term in Equation (5) equals zero because
the electric field density E = 0. The second term expresses the magnetic force
whose direction is perpendicular both to velocity v of the charge q and to magnetic field B. The force
action depends upon the charge and the magnitude of so called cross product of v × B,
i.e. the velocity and flux density vectors, where the relative directions of
these two vectors are taken into account. The magnitude of the force equals qvB sinϕ, where ϕ is the angle between v and B. If the angle ϕ = 90o, i.e. v is perpendicular to B, the particle trajectory is
circular with a radius of r = mv/qB.
For angles ϕ smaller than 90° the charge moves along a helix with the axis
parallel to the field lines. Obviously, if ϕ= 0o no action of
magnetic force is observed. Silva et al. [31] taking v @ 0.992 m/s
(determined experimentally) and q =
3.2 ×10−19
C (divalent cation) in the field B = 1T calculated the Lorentz force to be 3.17×10-19 N. Because the
ion mass is equal to 10-25-10-26 kg, the acceleration
(F/m) can be as large as 106-107 m/s2 which
can cause the ion polarization. In
our experiments the MF in the ring magnet changes radially from the top inner
edge to its center from 0.347 T to 0.053 T, which occurs on the distance of 19
mm. Hence ¶B/¶x on the sample surface level
equals to 43.2 T/m and 7.9 T/m, respectively. Then the MF gradient changes from
14.96 T2/m to 0.42 T2/m, respectively [13]. Because of
the field gradient and some mixing during the samples weighing every 30 min,
the ions moves in the solution. Let us assume a v value 0.5 m/s and if
some of the ions cross perpendicularly the field lines, the Lorentz force F = qvB for a monovalent ion amounts to
(1.6×10-19 C × 0.5 m/s × 0.347 T) = 0.278×10-19 N. Hence
the acceleration force F/m acting on the dodecylsufate ion C12-O-SO3-
(4.406×10-25 kg/ion) would be 6.3×104 m/s2 and
that acting on C12-N(CH3)3+ (3.79
×10-25 kg/ion) would amount to 7.3 ´
104 m/s2. The force at the magnet center is ca. 6.5 times
lower than those at the edge. Although above calculations are very rough ones
they show possible way to understand the observed MF effects. Conclusions These
preliminary experiments showed for the first time that in a common laboratory
environment preserving comparable conditions of temperature and humidity the
static MF effects on the evaporation of water from both cationic and anionic
surfactant solutions are present. Although they are not quantitatively
reproducible, they are reproducible qualitatively. Nevertheless the amount of
experiments is too small to be evaluated statistically the obtained changes can
be assumed as significant because in each experiment simultaneously with MF-
treated sample the reference MF-untreated sample was present. Thus in most
carried out experiments the MF affects evaporation of water from cationic and
anionic surfactant solutions. Water from the MF-treated samples evaporates
faster than that from the untreated ones thus leading to a larger evaporated
amount of water during the same time. Larger MF effect observed in the
experiments for the cationic than anionic surfactant solutions can be
understood by taking into account the different properties of the two head
groups, the anionic -O-SO3-Na+ and cationic -N+(CH3)Br-.
The cationic group is over 3 times larger than the anionic and possesses three
hydrophobic methyl groups -CH3. Also the Na+ counterions
are located at a larger distance from the head than Br-. The sulfate
group can form relatively strong hydrogen bonds with water molecules while the hydrogen bond with the
ammonium group is weak, if ever. These differences reflect in the observed
differences of the MF effects on these surfactant solutions. Generally, MF
increases evaporated amount of water from both surfactant solutions and the
mean relative values from several experiments are comparable for up to 150 min
of their duration. The rough calculations indicate that MF can interact both
perpendicularly to the liquid surface as the bulk magnetic force This
work was supported by Polish National Centre of Science, grant 2016/21
B/ST4/00987, which is greatly appreciated. 1.
Chibowski
E and Szcześ A. Magnetic water treatment - A review of the latest approaches
(2018) Chemosphere 203: 54-67. https://doi.org/10.1016/j.chemosphere.2018.03.160 2.
Guo
YZ, Yin DC, Cao HL, Shi JY, Zhang CY, et al. Evaporation rate of water as a
function of a magnetic field and field gradient (2012) Int J Mol Sci 13:
16916–16928. https://doi.org/10.3390/ijms131216916 3.
Toledo
EJL, Ramalho TC and Magriotis ZM. Influence of magnetic field on
physical-chemical properties of the liquid water: Insights from experimental
and theoretical models (2008) J Molecular Sci 888: 409–415. https://doi.org/10.1016/j.molstruc.2008.01.010 4.
Coey
JMD. Magnetic water treatment-how might it work? (2012) Philos Mag 92:
3857-3865. https://doi.org/10.1080/14786435.2012.685968 5.
Sammer
M, Kamp C, Paulitsch-Fuchs AH, Wexler AD, Cees J N, et al. Strong gradients in
weak magnetic fields induce DOLLOP formation in tap water (2016) Water 8: 79. https://doi.org/10.3390/w8030079 6.
Niu
XF, Du K and Xiao F. Experimental study on ammonia-water falling film
adsorption in external fields (2010) Int J Refrigeration 33: 686-94. https://doi.org/10.1016/j.ijrefrig.2009.11.014 7.
Nie
BS, Guo JH, Zhao H, Zhang JL and Hong T. Comparative effects of magnetic field
and surfactants on the surface tension of mine water (2013) Disaster Advances
6: 53-61. 8.
Soarez
PIP, Alves AMR, Pereira LCJ, Coutinho JT, Ferreira IMM, et al. Effects of
surfactants on magnetic properties of iron oxide colloids (2014) J Colloid
Interface Sci 419: 46-51. https://doi.org/10.1016/j.jcis.2013.12.045 9.
Haracz
S, Hilgendorff M, Rybka JD and Giersig M. Effect of surfactant for magnetic
properties of iron oxide nanoparticles (2015) Nuclear Instruments Methods Phys
Research B 364: 120 -126. https://doi.org/10.1016/j.nimb.2015.08.035 10.
Zhou
Q, Qin B, Wang J, Wang H and Wang F. Effect of preparation parameters on
wetting features of surfactant-magnetized water for dust control in Luwa mine
China (2018) Powder Technology 326: 7-15. https://doi.org/10.1016/j.powtec.2017.12.002 11.
Ivanković
T and Hrenović J. Surfactants in the environment (2010) J Surfactants
Environment 6: 95-110. https://doi.org/10.2478/10004-1254-61-2010-1943 12.
Olkowska
E, Polkowska Z and Namiesnik J. Analytics of Surfactants in the Environment:
Problems and Challenges (2011) Chem Rev 111: 5667-5700. https://doi.org/10.1021/cr100107g 13.
Nakagawa
J, Hirota N, Kitazawa K and Shoda M. Magnetic field enhancement of water
vaporization (1999) J Appl Phys 86: 2923-2925. https://doi.org/10.1063/1.371144 14.
Kitazawa
K, Ikezoe Y, Uetake H and Hirota N. Magnetic field effects on water, air and
powders (2001) Physica B, 294-295: 709-714. https://doi.org/10.1016/s0921-4526(00)00749-3 15.
Holysz
L, Szczes A and Chibowski E. Effects of a static magnetic field on water and
electrolyte solutions (2007) J Colloid Interface Sci 316: 996-1002. https://doi.org/10.1016/j.jcis.2007.08.026 16.
Szcześ
A, Chibowski E, Holysz L and Rafalski P. Effects of static magnetic field on
water at kinetic condition (2011) Chem Eng Process 5: 124-127. https://doi.org/10.1016/j.cep.2010.12.005 17.
Rashid
FL, Hassan NM, Jafar AM and Hashim A. Increasing water evaporation rate by
magnetic field (2013) Int Sci Invest J 2: 61-68. https://doi.org/10.1016/j.cep.2017.06.009 18.
Guo
YZ, Yin DC, Cao HL, Shi JY, Zhang CY, et al. Evaporation rate of water as a
function of a magnetic field and field gradient (2012) Int J Mol Sci 13:
16916-16928. https://doi.org/10.3390/ijms131216916 19.
Seyfi
A, Afzalzadeha R and Hajnorouzi A. Increase in water evaporation rate with
increase in static magnetic field perpendicular to water-air interface (2017)
Chem Eng Process 120: 195-200. https://doi.org/10.1016/j.cep.2017.06.009 20.
Amor
HB, Elaoud A, Salah NB and Elmoueddeb K. Effect of Magnetic Treatment on
Surface Tension and Water Evaporation (2017) Intern J Advan Ind Engin 5:
119-124. http://Dx.Doi.Org/10.14741/Ijae/5.3.4 21.
Chibowski
E, Szcześ A and Hołysz L. Influence of Magnetic Field on Evaporation Rate and
Surface Tension of Water (2018) Colloids Interfaces 2: 68. https://doi.org/10.3390/colloids2040068 22.
Van Der Waals
Interactions - Chemistry LibreTexts (accessed on April 30, 2018). 23.
Van
Oss CJ and Costanzo PM. Adhesion of anionic surfactants to polymer surfaces and
low energy materials (1992) J Adhesion Sci Technol 6: 477-487. https://doi.org/10.1163/156856192x00809 Magnetic field effects, Anionic and cationic
surfactant, Water evaporationMagnetic Field Effects on Aqueous Anionic and Cationic Surfactant Solutions Part I: Water Evaporation
Emil Chibowski and Aleksandra Szcześ
Abstract
Full-Text
However, to our knowledge only in few papers the investigations of MF effects
in different systems where a surfactant was present are reported [6-10].
However, no paper describes any MF effects on pure surfactant solutions. On the
other hand, surfactants are present in the surface and waste waters, soil, and
many industrial waters, sewage
treatment plants, laundry, etc. [11,12].
Therefore, it seemed us interesting to carry out study in a natural room
environment to learn whether some MF effects will appear in pure anionic or
cationic surfactant aqueous solutions, and if so, whether the effects are
reproducible qualitatively and/or quantitatively. In this paper first the MF
effects on the rate of water evaporation from a surfactant solution was
studied. The enhanced water evaporation from pure water was already reported in
some papers [13-20].Experimental
Results
and Discussion
Anionic
sodium dodecylsulfate, SDS, solution
For Equation 1 refer PDF
a2 -
is the surfactant activity in the bulk solution (concentration in the case of diluted
solutions). 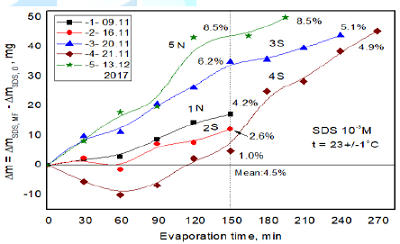
Cationic
dodecyltrimethylammonium bromide, DoTAB, solution
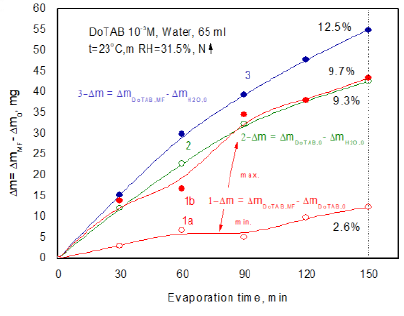
Curves:
1a and 1b-between magnetized and non-magnetized solutions, maximum and minimum
differences obtained; 2-between non-magnetized solution and pure water;
3-between magnetized solution and pure water.
First
of all, the size of these groups is different which is 0.17 nm2 for
SDS and 0.54 nm2 for the hard-core area of DoTAB. These areas were estimated
from knowledge of the bond lengths, bond angles and atomic volumes using a
molecular model of the headgroup [26]. Also the distance from the hydrophobic
core surface to the centre of the counterion location is 0.545 nm and 0.345 nm,
respectively [26]. Therefore the Na+ counterions are at a larger
distance from the -O-SO3- headgroup than Br-
from the -N+(CH3)3. The Critical Micelle
Concentration (CMC) of these surfactants at 25°C amounts to 8.2 mM
and 11.0 mM, respectively. Moreover, the SDS molecules -O-SO3-
group can form hydrogen bonds with water molecule oxygen atom but this is not
the case for DoTAB whose head is more hydrophobic because of the presence of
three methyl -CH3 groups and only a weak N--H hydrogen bond the -N+(CH3)3
group can form [27]. The determined surface tension of 10-3 M DoTAB
is 60.7 mN/m while that of SDS was 49.0 mN/m (will be publish in Part II of the
paper). Because the DoTAB head group is much larger than the SDS therefore it
can be expected that the same surface area occupies less DoTAB head groups -N+(CH3)3
than -O-SO3- of SDS. Moreover, the Br- counterions
are located closer to the head [26] and hence at the solution surface the
interactions between water molecules and the head group are much weaker than
those at the SDS solution surface. Therefore this cationic surfactant can also
reduce some of the hydrogen water-water
molecules bonding. In consequence evaporation of water from the 10-3 M
DoTAB solution is easier than from pure water (Figure 8), contrary to the SDS
solution (Figure 4).
Analogical equation can be written for water
molecules in the surface layer.
For Equation 3 refer PDFPossible
mechanisms of MF action
In
the magnetic field B = 8 T and the field gradient B·dB/dx = 320 T2/m the force
corresponded to as much as 17% of the gravitational force acting on the air
which can be compared to the thermal convection effect that would be caused by
50K temperature increase from 293K [13]. In the case of our experiment at the
water surface close to the inner magnet wall the gradient B·dB/dz amounted to 15 T2/m
but only 0.42 T2/m at the magnet centre (Figures 1b and 1c) [21].
Hence the maximum force difference (Equation
4) amounted to 0.089 N/kg which is only 0.91% of the gravitational force and
its contribution in the magnetic convection is rather minimal. Then the Lorentz
force acting on the ionic surfactant solutions can be analyzed.
For Equation 5refer PDF![]() , as well as horizontally as the Lorentz
force. In the Part II of this paper the MF effects on the surface tension of
these two surfactants solutions will be described. To our knowledge such MF
effects on the surfactant solutions are published for the first time in the
literature although a number of papers have been published on the MF effects on
water evaporation from pure water. These results suggest that more systematic
study at well-defined conditions are needed to better recognized these effects
and the MF mechanisms causing their appearance. Such study will be conducted
next. Potentially these MF effects may have a practical meaning in the
processes where water is evaporated from surfactant
solutions.
, as well as horizontally as the Lorentz
force. In the Part II of this paper the MF effects on the surface tension of
these two surfactants solutions will be described. To our knowledge such MF
effects on the surfactant solutions are published for the first time in the
literature although a number of papers have been published on the MF effects on
water evaporation from pure water. These results suggest that more systematic
study at well-defined conditions are needed to better recognized these effects
and the MF mechanisms causing their appearance. Such study will be conducted
next. Potentially these MF effects may have a practical meaning in the
processes where water is evaporated from surfactant
solutions.Acknowledgements
References
Keywords