Research Article :
Pier Maria Fornasari Based on Chinese CDCP report on COVID-19, 14% of
patients presented severe disease and 5% critical conditions. The average
case-fatality rate was 2.3%, but mortality was as high as 49% in patients with
critical illness. Serious life threatening thromboembolic complications have
been found in 71.4% of non-survivors and micro/macro angiopathic coagulopathy
has been found, at autopsy also, with highly increased neutrophil number,
fibrinogen, concentrations of D-dimer and FDPs and NETs, ATIII decrease and
normal number of platelets. A cytokine storm and interaction between
inflammation and coagulation has been advocated as explanation of
hypercoagulability. It has been shown that SARS-CoV-2 infection of alveolar
cells is driven by the S-protein by engaging ACE2 and TMPRSS2 cell receptors. Whose
activation depends on the activity of various host proteases. Full inhibition
of SARS-CoV-2 entry was observed when serine proteases inhibitor camostat
mesylate was coupled with Cathepsin B/L inhibitor E-64d. In addition multiple
proteases are involved in host immune response against viral invasion and
immunopathology related to imbalanced immune activation. In this paper it’s hypothesized
that the severity of Covid-19 is induced by recruitment of innate responder
neutrophils, which release proteases and NETs inducing endothelial damage and
imbalance of the four major proteolytic cascades (coagulation, complement,
fibrinolysis and kallikrein) with prevalence of activators over inhibitors and
consequent thrombotic complications. Platelets adhesion to damaged endothelium
and vWFVIII multimers presence, due to loss of ADAMTS13, contributes to
hypercoagulability state. Human plasma or serine protease inhibitors like
aprotinin can help to control neutrophil induced “proteolytic storm”. The goal
of this paper is to support the view that, in SARS-CoV-2 infection, proteases
have a key role and exceeding imbalanced neutrophil innate “unfriendly fire”
response can be identified as the trigger of a “proteolytic storm”, responsible
for subsequent well known hyper coagulation and “cytokine storm” and human
plasma, in adequate volumes, together with serine proteases inhibitors can be
an effective therapeutic strategy. According to the largest current
report from the Chinese Center for Disease Control and Prevention with 72 314
cases, 58 574 patients (81%) were classified as mild, 10 124 (14%) were
classified as severe, and 3616 (5%) were considered critical (respiratory
failure, septic shock, and/or multiple organ failure) [1]. Among 201 patients
in Wuhan, Wu, et al [2] reported that risk factors associated with development
of acute respiratory distress syndrome and death included older age,
neutrophilia, organ dysfunction, coagulopathy and elevated D-dimer levels. As
of November 24, 2020, John Hopkins Covid-19 dashboard has documented a total of
59.400.000 cases with over 1.400.000 deaths worldwide. The SARS-CoV-2 infection
is a protease dependent process as cell entry depends on the binding of the
Spike protein’s S1 subunit to ACE2 on the
target cell surface and host proteases, furin, as well as TMPRSS2 processes the
S protein to facilitate membrane fusion, allowing SARS-CoV-2 to have enhanced
proteolytic activation in a wider range of tissues [3]. The strategy used by
SARS-CoV-2 for cellular entry is the same in all the tissues Presenting
dual-positive ACE2+TMPRSS2+cells, employing its S protein, previously primed by
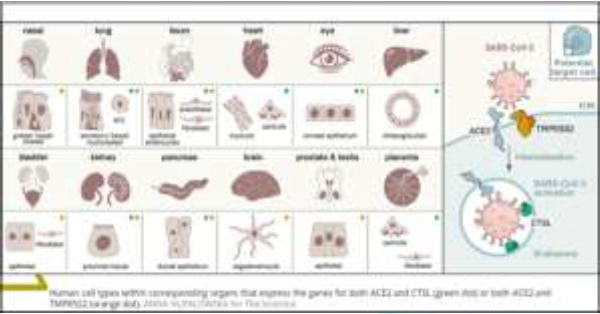
TMPRSS2 protease and cathepsin B/L, binding to ACE2 receptor [4-8]. ACE2, the
viral receptor, and one of its entry-associated proteases, TMPRSS2, are
expressed in nasal goblet cells, in lung goblet, multiciliated and AT2 cells
and gut epithelial enterocytes, in pancreatic ductal cells, bladder, testis,
prostate and kidney epithelial cells, cholangiocytes, oligodendrocytes in the
brain, inhibitory enteric neurons, heart fibroblasts/pericytes, and fibroblasts
and pericytes in multiple other tissues (Figure1).
In line with the kidney’s role in the renin-angiotensin-aldosterone system,
dual-positive cells are enriched in the proximal tubular cells and in principal
cells of the collecting duct [9]. A recent paper has shown that therapeutic
aprotinin concentrations inhibit SARS-CoV-2 replication as entry inhibitors and
by compensating for down regulated cellular protease inhibitors during later
replication cycles [10]. Aprotinin aerosol, as approved in Russia for the
treatment of influenza, may be a particularly promising strategy to suppress
virus replication and thus prevent Covid-19 lung injury [11]. Inverse correlation between disease severity and lymphopenia
has been observed according to Tan L, et al. [12] critical patients with
lymphocyte percentage <5% were more likely to become critically ill, with
need for intensive care therapy and high mortality rate. Along the same line,
imbalance between interferon production and chemokines and an “eicosanoid
storm” have been found. The physiological response to virus infection starts at
intracellular replication, through Pattern Recognition Receptors (PRRs) and
transcription factors (Interferon Regulatory Factors (IRFs) and nuclear factor
kB) activation, inducing cellular antiviral defenses (IFN-I and IFN-III,
respectively) and subsequent up regulation of ISGs and leukocytes recruitment
by chemokine secretion [13-15]. Imbalance between Interferon and Chemokines: Early interferon antiviral activity is depressed, while
innate immunity neutrophils are highly recruited. The infected cells delay the
IFN-I and -III response by inhibiting innate immune signaling, induce up
regulation of chemo attractants for neutrophils and monocytes (HMGB1, CCL2,
CCL8 and CXCL family) cytokines storm (IL-2R, IL1RA, IL-6, IL-8, IL-10 and TNF)
and increased reactants biomarkers (Example: procalcitonin, serum ferritin, and
C-reactive protein) [16]. The combination of TNF-α and IFN-γ induced
inflammatory cell death characterized by pyroptosis, apoptosis, and necroptosis
(PANoptosis). Mechanistically, TNF-α and IFN-γ co-treatment activated the
JAK/STAT1/IRF1 axis, inducing nitric oxide production and driving
caspase8/FADD-mediated PANoptosis. TNF-α and IFN-γ caused a lethal cytokine
shock in mice that mirrors the tissue damage and inflammation of COVID-19 and
inhibiting PANoptosis protected mice from this pathology and death [17]. Proteomic and metabolomic studies showed activation of
complement pathways, acute phase reactants (C-Reactive Protein and Serum
Amyloid proteins SAA1 and SAA2), proteins implicated in interleukin IL-6 signaling,
Inter-α-Trypsin Inhibitor Heavy Chain 4 (ITIH4), Haptoglobin (HP), Leucine-Rich
Alpha-2-Glycoprotein (LRG1), Monocyte differentiation antigen CD14 and the
Liposaccharide Binding Protein (LBP), known to induce IL-6 expression. Thus,
the proteomic approach surprisingly revealed a very IL-6 centered response. SARS-Cov-2
infected lung cells also overexpressed complement activation genes, involved in
neutrophil degranulation, with deposits of terminal complement components
C5b-9, C4d and MASP2 attacking the host ECs and causing transmembrane channel
formation on the endothelium and inducing endotheliopathy, involved in ARDS
like syndrome with systemic inflammation and lung neutrophilia [18]. An up
regulation of fibrinogen, Protein Z-Dependent Protease Inhibitor and SERPINA10
was found, further highlighting the importance of coagulation in SARS-CoV-2
infection. In severe COVID-19 lung infection, a catastrophic microvascular
injury syndrome is caused by activation of complement and coagulation pathways,
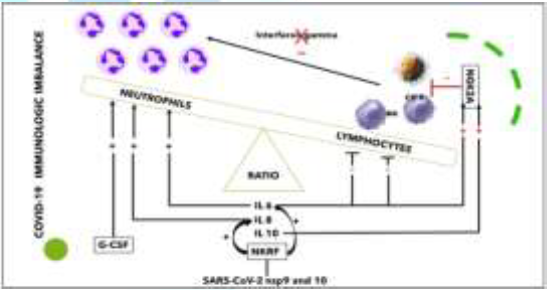
inducing an associated procoagulant state [18]. Imbalance between Innate Immunity Cells: Qin, et al. [19] described the occurrence of a dysregulated
immune response with a marked decrease in T-cell number, higher leukocyte
counts and Neutrophil-To-Lymphocyte Ratio (NLR), as well as lower percentages
of monocytes, eosinophils, and basophils. SARS-CoV-2 nsp9 and nsp10 directly
target NKRF to facilitate IL-8/IL-6 production and thus the response is
imbalanced versus activation of the innate immune response [16]. Innate immune
response plays an important protective or destructive role, depending on the
progression of the disease. The association between human serine proteases
trypsin, thrombin, tryptase, and elastase with increased expression of MCP-1
has been shown and the inhibition of these proteases resulted in the inhibition
of MCP-1 secretion via inactivation of various Protease-Activated Receptors
(PARs) [20]. Furthermore, neutrophils respond to viral infections by activation
of neutrophil elastase, Cathepsin G, and proteinase 3, playing roles both
intracellularly and extracellularly [21,22]. However, the presence of a high
number of neutrophils at the inflammation site could correspond to imbalanced
protease activity. This further leads to various inflammatory disorders, tissue
damage, lung dystrophy, ARDS, and potentially death [22] (Figure 2). Figure 2: Innate immunity cells imbalance (personally modified by Antonioli L, et al. [23]. Several cytokines, such as IL6 and IL-10, were shown to
upregulate NKG2A expression and consequent lymphocyte downregulation, while
IL-6 and IL-8 impair the functions of NK cells via STAT3-dependent mechanisms.
NKG2A, thus, is a key factor in the altered balance between neutrophils and
lymphocytes [23] (Figure 2). High levels of IL-6, IL-8 and G-CSF enhance
neutrophil recruitment and express an inhibitory action on NK cells further
reducing interferon (IFN)-γ production [24-26]. Recent studies and autopsy
results have confirmed infection and destruction of lymphocytes in the spleen,
lymph nodes, and lymphoid tissues of the gut [26,27]. In these sites
lymphocytes were reduced markedly in germinal centers and in situ hybridization
detected SARS viral positivity in the residual immune cells in the spleen and
in circulation. Respiratory Tract Infection, Neutrophilia, Proteolytic Cascades
and Platelets Activation Resulting in Inflammatory Endotheliopathic,
Microangiopathy: Focusing on the areas of the respiratory tract involved and
based on the cells that are likely infected, COVID-19 can be divided into three
phases that correspond to different clinical stages of the disease. Stage 1: Asymptomatic state (Initial 1-2 days of infection): inhaled
virus SARS-CoV-2 likely binds to epithelial cells in the nasal cavity and
starts replicating. The viral burden may be low. Stage 2: Upper airway and conducting airway response (Next few
days): virus propagates and migrates down the respiratory tract along the
conducting airways. Stage 3: Alveolar phase. The disease COVID-19 is clinically
manifest. About 19% of the infected patients will progress to stage 3 diseases
and will develop pulmonary infiltrates. The virus infects alveolar type II
cells. SARSCoV-2 propagates within type II cells, large numbers of viral
particles are released and the cells undergo apoptosis and die. The alveolar phase is evolving in 2
different patterns: 3a) Moderate
with absent or minor endothelial leakage, 3b) Severe with alveolar collapse due
to surfactant loss, fluid filling of interstitium, engulfing protein-rich fluid
with neutrophils release products like NETs, reduced gas exchange, endothelial
lesion, through which SARS-CoV-2 virus can enter into the bloodstream and
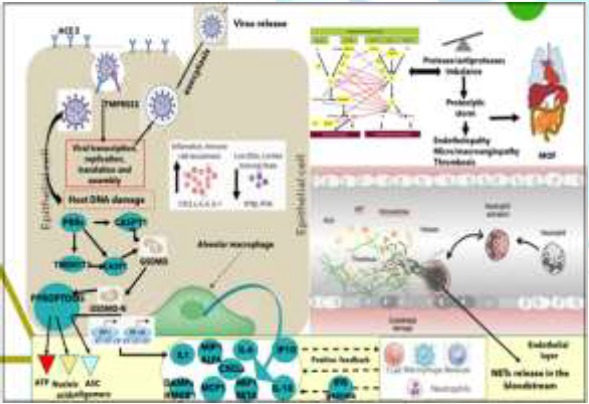
induce viral sepsis. In SARS-Cov-2 infected lung cells, deposits of terminal
complement components C5b-9, C4d and MASP2 attack the host ECs and cause transmembrane
channel formation on the endothelium and induce endotheliopathy, involved in
ARDS like syndrome with systemic inflammation and lung neutrophilia [28]. A
similar pattern has been shown in purpuric skin lesions due to a
pauci-inflammatory thrombogenic vasculopathy, with co-localization of COVID-19
spike glycoproteins and deposition of C5b-9 and C4d in both grossly involved
and normally-appearing skin and in lung interalveolar septa [29]. Platelets
also play a critical role in lung innate defense response, being lung a primary
site for platelet biogenesis: activated platelets engulf virions and secrete
antiviral molecules (example: a-granules) to destroy virions, HMGB1 and express
surface P-selectin enabling the initial attachment of neutrophils from the
bloodstream [29] (Figure 3). The Neutrophils “friendly fire”, the NETosis and the
Proteolytic Storm. Dangerous not inhibited partnership of platelets, complement
and coagulation cascades for endotheliopathy and thrombogenesis: The lung alveoli are thus the last
defense line against SARS-Cov-2 bloodstream dissemination and viral sepsis and
thus innate immunology is highly involved in fighting. In severe COVID-19,
neutrophils, together with other mononuclear, are the first cells of the immune
system to migrate to the infected alveoli, recruited by interferons, IL-1β and
IL-6, where they attack SARS-CoV-2, but in addition to direct virus-inflicted
pathologies, their exaggerated “unfriendly fire” responses, resulting in a
“proteolytic storm” and, inducing alveolar capillary endotheliopathy,
contribute to disease severity and subsequent viral dissemination [16,30]. The
mechanisms that neutrophils undertake for host defense are phagocytosis,
degranulation, cytokine production and Neutrophil Extracellular Traps (NETs)
release, known as NETosis. Neutrophil extracellular traps are DNA structures
released including histones and over 30 components of primary and secondary
granules, such as elastase, myeloperoxidase, cathepsin G (CG), lactoferrin,
pentraxin 3, gelatinase, proteinase 3, LL37 and peptidoglycan-binding proteins.
Three models for NETosis are known to date: suicidal (NETs release and
neutrophil lysis), vital NETosis (triggered by TLRs stimuli, platelet
glycoprotein Ib, complement activation, after release neutrophils are still
able to phagocytose pathogens and have a normal lifespan) and mitochondrial DNA
is released instead of nuclear DNA [31]. Platelet HMGB1 protein (passively
released extracellularly as a prototypical DAMP from dying cells or stressed or
activated cells present in any tissue) is the major endogenous inducer of NETs
formation. NET chromatin disrupts epithelial lining, induces platelet
aggregation and activates further neutrophils recruitment [32]. NETs, via
electrostatic interactions, activate the contact pathway of coagulation and,
through tissue factor, the intrinsic pathway [30,33]. NETs form a scaffold for thrombus
formation by promoting platelet adhesion and by concentrating coagulation
factors involved in clotting. Thrombus-resident neutrophils are strategic for
thrombi extension by binding factor XII and supporting its activation through
NETosis [34]. The endothelial cells damage (endotheliopathy), induced by
SARS-CoV-2 virus and neutrophil elastase, triggers the activation of two
independent endothelial pathways (inflammatory and microthrombotic), through
release of inflammatory cytokines (interleukin IL-1, IL-6, tumor necrosis
factor-α, and others) and activation of the platelet and endothelial exocytosis
of ULVWF, mediating microthrombogenesis via “activation of microthrombotic
pathway”. In parallel endothelial damage inhibits ADAMTS13 biosynthesis, while
Neutrophil Elastase proteolytically cleaves and significantly decreases its
plasma level [35]. FXIa and α-thrombin remove
C-terminal domain of ADAMTS13, blocking its ability to cleave VWF on the
endothelial cell surface, and increase the release of VWF antigen by
endothelial cells, resulting in persistence of VWF strands and causing an
increase in platelet adhesion under flow conditions [36]. This pathological
chain of events event has been described also for multisystemic vasculitis in
Kawasaki Disease, characterized by platelet stimulation with increase in the
shedding of Pselectin, translocating at the surface and externalized with
subsequent hyperactivation, and the detection of circulating platelets-neutrophils
aggregates. Neutrophils recruited by platelets Pselectin and PSGL-1 (vascular
adhesion molecules playing an important role in the inflammatory response by
mediating the interaction of leucocytes with stimulated endothelium and
platelets bound in the vicinity of vascular injury) contribute, through
Toll-like receptor 4, to NETs formation, NETs cause platelet activation and
aggregation, thus linking inflammation and thrombosis to support the relevance
of this mechanism in the pathogenesis of Covid-19. Simultaneously, serine
proteases released by neutrophils and present in NETs cleave coagulation
inhibitors such as tissue factor pathway inhibitor and antithrombin [37]. On the damaged endothelial surface
NETs, ULVWF multimers, platelets and activated not inhibited clotting and
complement pathways initiate thrombogenesis within the microvasculature,
leading to microthrombi enriched also by leukocytes recruited in the P-selectin
dependent manner [38]. The microthrombi can become sufficiently large to be released
from endothelial cells into the circulation, resulting embolism [39].This
condition can be recalled “TTP-like syndrome” [40]. Blood circulation action
and mechanical stress (like forced ventilation) may be sufficient to physically
disrupt the fragile structure of NETs in the bloodstream, releasing NET
fragments. Mechanically disrupted NETs augment NETosis and NETosis propagates
inflammatory response. TLR inhibitors may reduce inflammation, specifically by
preventing NET-induced NETosis. Intravascular NETosis is thus responsible for
initiation, dissemination and local accretion of thrombotic events in arteries,
veins and in the microvasculature, with end-organ damage in lungs, heart,
kidneys and other organs [41]. Through endothelial lesions and
NETs, SARS-CoV-2 virus activates its viremic phase and in each interested organ
(Figure 1) follows the same cellular entry strategy and catastrophic
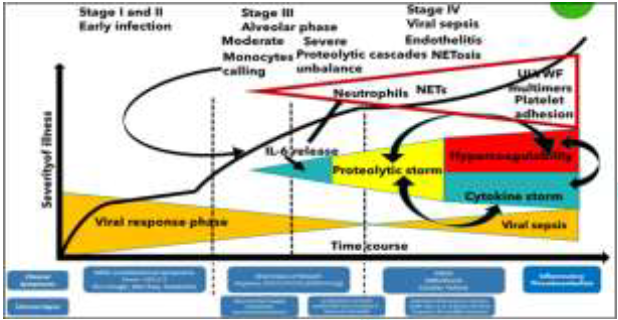
microvascular injury syndrome, causing final MOF. Proteolytic cascades balance between activators and
inhibitors is unbalanced in Covid-19: If NETs induce hypercoagulability,
the significant increase in neutrophil numbers and the released proteolytic
enzymes (mainly elastase) contribute to a consumption of proteases inhibitors,
with an umbalance of physiologic conditions and instauration of the
“proteolytic storm” [42] (Figure 4). The activators/inhibitors balance in
proteolytic cascades is essential for homeostasis and, due to this, in
normality the inhibitor plate is largely superior to activator one [43-45]. The
innate serine protease system has four major columns, coagulation,
fibrinolysis, kallikrein and complement [44]. These systems are strictly
correlated, interconnected and their physiological mantainance is the result of
a rigorous balance. Complement directly enhances coagulation and, in addition,
inhibits anticoagulant factors, while certain coagulation enzymes activate
complement components. The interplay between complement and coagulation is
crucial to understand the clinical implications in Covid-19, in which
complement-coagulation interactions contribute to the development of
life-threatening complications [23]. The contact system, also named as plasma
kallikrein-kinin system, consists of three serine proteinases: coagulation
factors XII and XI, plasma prekallikrein and high molecular weight kininogen.
Once activated by NETs, this system is prothrombotic by activating intrinsic
pathway and proinflammatory by producing bioactive peptide bradykinin.
Extrinsic and intrinsic pathway of blood coagulation induces simultaneous
activation of the complement and fibrinolysis cascades, with an extensive cross
talk mutually fine-tuning their activation status [46]. Main family of the serine protease
inhibitors (SERPINs) is formed by SERPINA1 (ɑ1-antitrypsin) protecting lung
tissue from neutrophil elastase, SERPINA5 (Protein C inhibitor), SERPINC1 (also
known as antithrombin) controls coagulation proteases, SERPIND (Heparin
cofactorII), SERPINE1 (plaminogen activator inhibitor 1), SERPING1 (also known
as plasma C1 inhibitor) regulates complement, callicrein and contact phase
activation and SERPINF2 (also known as ɑ-2- antiplasmin) inhibits plasmin and
regulates fibrinolysis. Complement activation is inhibited also by
Decay-accelerating factor (DAF) and Factor H (alternative pathway). Alpha 2
macroglobulin acts as an antiprotease for a variety of proteases like plasmin,
kallikrein and thrombin. A delicate balance between serine proteases and their
serpin inhibitors is crucial for normal functioning of biological pathways [43].
Proteases/antiproteases balance is present also at the endothelial surface,
where thrombomodulin, forming complexes with thrombin, induces protein C
activation to suppress blood coagulation, while TNF-alpha and IL-1beta,
inducing TF and PAI-1, down-regulate the expression of thrombomodulin.
Procoagulant TF upregulation with downregulation of the anticoagulant
TM/Protein C system converts the normal anticoagulant endothelium into a
prothrombotic endothelium. Lastly, NETs triggered significant platelet
aggregation. A proteases/antiproteases balance is present also at alveolar
space, where SERPINA134 strongly and specifically inhibits neutrophil elastase.
When the inhibitor concentration is sufficient to block released elastase, no
lesion happens nor in alveolar epithelium nor in alveolar endothelial wall and
this corresponds with the moderate Covid-19 clinical condition. Otherwise, if
SERPINA135 is overhelmed or is absent/deficient, as in homo/eterozygous
patients (about 4% European population), imbalance between elastase and
anti-elastase activity, free elastase causes progressive damage of both alveoli
and endothelium, inducing endotheliopathy and thrombogenic state previously
described. The hypercoagulability, as unbalance of proteases/antiproteases
cascades, the decrease of ADAMTS13, the endotheliopathy, the increased platelet
activation, the ULVWF multimers and the NETosis together create a severe
thromboembolic environment, similar to Thrombotic Thrombocytopenic Purpura/HUS
conditions and Acute Promyelocytic Leukemia activation of clotting systems with
secondary hyperfibrinolysis [45,47,48]. Critical SARS-CoV-2 infection, severe thrombosis,
“proteolytic” and “immunological” storm: the possible role of human
convalescent/non convalescent plasma and of serine proteases inhibitors as
“firehose”, due to proteolysis inhibitors support: Convalescent Plasma (CP) use in the
therapy of untreatable infectious diseases has been extensively but anecdotally
documented, including spanish Influenza A (H1N1) infections in 1915 to 1917, Severe
Acute Respiratory Syndrome (SARS) in 2003, pandemic 2009 influenza A (H1N1),
avian influenza A (H5N1) and several hemorrhagic fevers such as Ebola. Based on
studies, showing convalescent plasma antibodies can limit the virus
reproduction, CP has been considered for critically sick COVID‐19 patients [49].
In SARS-CoV and MERS, CP was shown to provide NAbs binding to Spike1-Receptor
Binding Protein (S1-RBD), S1-N-terminal domain and S2, thus blocking entry and
containing viral amplification. Very recently, Cochrane Database of
Systematic Reviews published a rapid review on convalescent plasma or
hyperimmune immunoglobulin for people with COVID-19 [50]. Including 19 studies
(2 RCTs, 8 controlled NRSIs, 9 non‐controlled NRSIs) with 38,160 participants,
of whom 36,081 received convalescent plasma. Two completed RCTs are awaiting
assessment (published after 19 August 2020). Were identified a further 138
ongoing studies evaluating convalescent plasma or hyperimmune immunoglobulin,
of which 73 are randomised (3 reported in a study registry as already being
completed, but without results). No completed studies evaluating hyperimmune
immunoglobulin was identified. The review also includes results from two RCTs
(both stopped early) with 189 participants, of whom 95 received convalescent
plasma. Control groups received standard care at time of treatment without
convalescent plasma. The conclusions of the review are
uncertain whether convalescent plasma decreases all‐cause mortality at hospital
discharge (Risk Ratio (RR) 0.55, 95% confidence interval (CI) 0.22 to 1.34; 1
RCT, 86 participants; low‐certainty evidence) and whether convalescent plasma
decreases mortality (time to event) (Hazard Ratio (HR) 0.64, 95% CI 0.33 to
1.25; 2 RCTs, 189 participants; low‐certainty evidence). Convalescent plasma
may result in little to no difference in improvement of clinical symptoms (i.e.
need for respiratory support) at seven days (RR 0.98, 95% CI 0.30 to 3.19; 1
RCT, 103 participants; low‐certainty evidence). Convalescent plasma may increase
improvement of clinical symptoms at up to 15 days (RR 1.34, 95% CI 0.85 to
2.11; 2 RCTs, 189 participants; low‐certainty evidence), and at up to 30 days
(RR 1.13, 95% CI 0.88 to 1.43; 2 studies, 188 participants; low‐certainty
evidence). No studies reported on quality of
life. Reporting of safety data and duration of follow‐up was variable. The
controlled studies reported on Adverse Events (AEs) and Severe Adverse Events
(SAEs) only in participants receiving convalescent plasma. Some, but not all,
studies included death as a SAE. The studies did not report the grade of AEs.
Fourteen studies (566 participants) reported on AEs of possible grade 3 or 4
severity. The majority of these AEs were
allergic or respiratory events. The studies are very uncertain whether
convalescent plasma therapy affects the risk of moderate to severe AEs (very
low‐certainty evidence). 17 studies (35,944 participants) assessed SAEs for
20,622 of its participants. The majority of participants were from one non‐controlled
NRSI (20,000 participants), which reported on SAEs within the first four hours
and within an additional seven days after transfusion. There were 63 deaths, 12
were possibly and one was probably related to transfusion. There were 146 SAEs
within four hours and 1136 SAEs within seven days post‐transfusion. These were
predominantly allergic or respiratory, thrombotic or thromboembolic and cardiac
events. A recently published randomized
clinical trial published by PlasmAr Study Group has concluded that no
significant differences were observed in clinical status or overall mortality
between patients treated with convalescent plasma and those who received
placebo [51]. All these studies were using not more than 500 ml of convalescent
plasma and the scientific reason for the transfusion was the activity of
neutralising antibodies against SARS-CoV-2. In this paper, on the contrary, we
have shown that the worsening of Covid-19 clinical symptoms is due to the
catastrophic “unfriendly fire” of recruited neutrophils, with overhelming and
imbalancing of serine proteolytic cascades activator proteases over inhibitors,
serious endotheliopathy, NETosis, ULVWF release, hypercoagulability and diffuse
micro/macrothrombi formation. This condition in the paper has been described as
“proteolytic storm”, which advances and sustains/is sustained by the well-known
“cytokine storm”as shown in Figure 4. Following this hypothesis, human
plasma, non-convalescent, should be used in adequate quantities (more than 2
liters and/or following plasma-exchange procedures) as it supplies: ·
Other antibodies able to mediate/neutralize pathways such as
complement activation, antibody-dependent cellular cytotoxicity and/or
phagocytosis [52,53]. Limiting immune complexes formation and cytokine release
such as IL-1β and TNFα ·
SERPIN family serine proteases inhibitors of the four
interconnected proteolytic cascades (clotting, complement, fibrinolysis and kallikrein) ·
ADAMTS13 supply, which cleaves ULVWF, reducing
hypercoagulability and thrombogenicity [54] ·
SERPINA1 inhibiting neutrophil elastase deleterious effects,
mainly at alveolo-capillary level [46] ·
SERPING1 counteracts platelet activation activity on clotting
and complement cascade45 and Pselectin/HGMB1 expression [13,38] ·
Other aspecific inhibitors like Alfa2 Macroglobulin and
Thrombomodulin
·
CP NAbs boost a much stronger immune response of newly
dendritic cells inf-cDC2 [55]. The NSP5 main protease (Mpro, 3C) is
investigated as a potential drug target because of its involvement in
processing the proteins coded from viral RNA [56]. Aprotinin can be used as a
treatment startegy to support serine proteases activators/inhibitors balance. The SARS-CoV-2 infection is a
protease dependent process as cell entry depends on the binding of the Spike
protein’s S1 subunit to ACE2 on the target cell surface and host proteases,
furin, as well as TMPRSS2 processes the S protein to facilitate membrane fusion,
allowing SARS-CoV-2 to have enhanced proteolytic activation in a wider range of
tissues [57]. Aprotinin have been shown to inhibit
SARS-CoV-2 entry and replication and thus an aerosol treatment as for influenza
is suggested. After cell entry, SARS-CoV-2 induces a profound immunological
unbalance, with a delay of the IFN-I and -III response and upregulation of
chemoattractants for neutrophils and monocytes, cytokines storm and increased
reactants biomarkers. A PANoptosis process is caused by the combination of
TNF-α and IFN-γ. Chemoattractants and cytokines enhance recruitment of
neutrophils, while suppressing lymphocytes with an increase of NLR, which is
considered a marker of severe Covid-19. Neutrophils, together with other
mononuclear, migrate to the infected alveoli, where they attack SARS-CoV-2, but
in addition to direct virus-inflicted pathologies, their exaggerated
“unfriendly fire” responses, resulting in a “proteolytic storm” and, inducing
alveolar capillary endotheliopathy, contribute to disease severity and
subsequent viral dissemination. One of the mechanisms that
neutrophils undertake for host defense is NETs release, known as NETosis. NETs,
via electrostatic interactions, activate the contact pathway of coagulation
and, through tissue factor, the intrinsic pathway and form a scaffold for
thrombus formation by promoting platelet adhesion and by concentrating
coagulation factors involved in clotting. Concurrently SARS-CoV-2, together
with neutrophil elastase and other proteases, induces endothelial cells damage
(endotheliopathy) triggering the activation of two independent endothelial
pathways and activation of the platelet and endothelial exocytosis of ULVWF
(due to ADAMTS13 biosynthesis inhibition), mediating microthrombogenesis and hypercoagulability
state. The activation of platelets and clotting proteolytic system involves the
other innate serine protease systems with their four major columns,
coagulation, fibrinolysis, kallikrein and complement, strictly correlated and
interconnected and their physiological maintenance is the result of a rigorous
balance between activators and inhibitors. The interplay between complement and
coagulation is crucial to understand the clinical implications in Covid-19. NETs
activate Kallikrein/kinin, inducing a prothrombotic state by activating
intrinsic pathway and proinflammatory state by producing bioactive peptide
bradykinin. Main family of the serine protease inhibitors (SERPINs) formed by
SERPINA1, SERPINA5, SERPINC1, SERPIND, SERPINE1, SERPING1 and SERPINF2 inhibit
the major serine protease cascades. Complement activation is inhibited also by Decay-Accelerating
Factor (DAF) and Factor H (alternative pathway). Alpha 2 macroglobulin acts as
an antiprotease for a variety of proteases like plasmin, kallikrein and
thrombin. A delicate balance between serine proteases and their serpin
inhibitors is crucial for normal functioning of biological pathways.
Proteases/antiproteases balance is present also at the endothelial surface
(thrombomodulin induces protein C activation to suppress blood coagulation) and
also at alveolar space, where SERPINA1 strongly and specifically inhibits
neutrophil elastase. When the inhibitor concentration is
sufficient to block released elastase, no lesion happens nor in alveolar
epithelium nor in alveolar endothelial wall and this corresponds with the
moderate Covid-19 clinical condition. If SERPINA1 is overhelmed or is
absent/deficient imbalance between elastase and anti-elastase activity causes
progressive damage of alveoli and endothelium, inducing endotheliopathy and
thrombogenic state previously described. Convalescent plasma has been recently
shown uneffective in several clinical trials with no significant differences
observed in clinical status or overall mortality between patients treated with
convalescent plasma and those who received placebo. All these studies were
using not more than 500 ml of convalescent plasma and the scientific reason for
the transfusion was the activity of neutralising antibodies against SARS-CoV-2.
In this paper, on the contrary, it has been shown that the worsening of
Covid-19 clinical symptoms is due to the catastrophic “unfriendly fire” of
recruited neutrophils, with over helming and imbalancing of serine proteolytic
cascades activator proteases over inhibitors, serious endotheliopathy, NETosis,
ULVWF release, hypercoagulability and diffuse micro/macrothrombi formation
(proteolytic storm). Thus, the transfusion of human plasma, non-convalescent,
should be used in adequate quantities (more than 2 liters and/or following
plasma-exchange procedures) due to the large quantity of protease inhibitors
supplied. Other serine protease inhibitors like aprotinin can be used to
restore serine protease balance, working as a “firehose” extinguishing “proteolytic
storm”.
SARS-CoV-2 cell entry is a protease
dependent process and can be efficiently inhibited by Aprotinin, a serine
protease inhibitor. Severe Covid-19 is the consequence of neutrophils induced
“proteolytic storm”, with serine proteases released in large quantities able to
overwhelm physiological homeostasis between activators and inhibitors. CP
transfused to supply NAbs against SARS-CoV-2 in severe Covid-19 patients has
failed in several clinical trials showing no significant differences in
clinical status or overall mortality between patients treated with CP and those
who received placebo and the median volume of infused CP was 500 m. CP supply
NAbs could be useful in the previous phases of SARS-CoV-2 infection, but
clinical trials with this target are absent. On the contrary, if CP or human
plasma and aprotinin are infused to supply serine protease inhibitors as a
“fire extinguisher” for neutrophils “unfriendly fire” and its consequences of
“proteolytic storm”, hypercoagulability, thrombosis and sepsis with MOF, the
infused volume should be at least 2000 ml or plasma-exchange should be
performed. Clinical trials are needed to confirm this hypothesis. 1. Wu Z and Mc Googan JM. Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention (2020) JAMA 323: 1239-1242. https://doi.org/10.1001/jama.2020.2648 2. Wu C, Chen X and Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China (2020) JAMA Intern Med 180: 934-943. https://doi.org/10.1001/jamainternmed.2020.0994 3. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, et al. Structure, function, and antigenicity of the SARSCoV-2 spike glycoprotein (2020) Cell 183: 281-292. https://doi.org/10.1016/j.cell.2020.11.032 4. Hoffmann, kleine-weiber H, shroeder S, kruger N, Herrler T, et al. Sars-cov-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor (2020) Fac opin 181: 271-280. https://doi.org/10.3410/f.737494462.793575061 5. Seth S, Batra J and Srinivasan S. COVID-19: Targeting Proteases in Viral Invasion and Host Immune Response (2020) Front Mol Biosci 7: 215. https://doi.org/10.3389/fmolb.2020.00215 6. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2 (2020) 581: 221-224. https://doi.org/10.1038/s41586-020-2179-y 7. Wang Q, Zhang Y, Wu L, Niu S, Song C, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2 (2020) Cell 181: 894-904. 8. Wan Y, Shang J, Graham R, Baric RS and Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus (2020) J Virol 94:e00127-20. https://doi.org/10.1128/jvi.00127-20 9. Muus C, Luecken MD, Eraslan G, Waghray A, Heimberg G, et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells (2020) CSH press, United States. https://doi.org/10.1101/2020.04.19.049254 10. Bojkova D, Bechtel M, McLaughlin KM, McGreig JE, Klann K, et al. Aprotinin inhibits sars-cov-2 replication (2020) 9: 2377. https://doi.org/10.3390/cells9112377 11. Zhirnov OP, Klenk HD and Wright PF. Aprotinin and similar protease inhibitors as drugs against influenza (2011) Antiviral Res 92: 27-36. https://doi.org/10.1016/j.antiviral.2011.07.014 12. Tan L, Wang Q, Zhang D, Ding J, Huang Q, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study (2020) Sig Transduct Target Ther. https://doi.org/10.1038/s41392-020-0148-4 13. Janeway CA and Medzhitov R. Innate immune recognition (2002) Annu Rev Immunol 20: 197-216. 14. Hur S. Double-stranded RNA sensors and modulators in innate immunity (2019) Annu Rev Immunol 37: 349-375. https://doi.org/10.1146/annurev-immunol-042718-041356 15. Sokol CL and Luster AD. The chemokine system in innate immunity (2015) CSH press, United States. https://doi.org/10.1101/cshperspect.a016303 16. Li J, Guo M, Tian X, Liu C, Wang X, et al. Virus-host interactome and proteomic survey of PMBCs from COVID-19 patients reveal potential virulence factors influencing SARS-CoV-2 pathogenesis (2020) CSH press, United States. https://doi.org/10.1101/2020.03.31.019216 17. Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes (2020) bioRxiv. https://doi.org/10.1101/2020.10.29.361048 18. Blanco-Melo D, Nilsson-payant BE, Liu WC, Uhl S, Hoagland D, et al. Imbalanced host response to sars-cov-2 drives development of covid-19 (2020) Cell press, United States. https://doi.org/10.1016/j.cell.2020.04.026 19. Qin C, Zhou L, Hu Z, Zhang S, Yang S, et al. Dysregulation of immune response in patients with coronavirus 2019 (covid-19) in wuhan, china (2020) clin infe diseases 71: 762-768. https://doi.org/10.1093/cid/ciaa248 20. Wang H, Yi T, Zheng Y and He S. Induction of monocyte chemoattractant protein-1 release from A549 cells by agonists of protease-activated receptor-1 and -2 (2007) European J Cell Biology 86: 233-242. 21. Méthot N, Rubin J, Guay D, Beaulieu C, Ethier D, et al. Inhibition of the activation of multiple serine proteases with a cathepsin C inhibitor requires sustained exposure to prevent pro-enzyme processing (2007) J Biol Chem 282: 20836-20846. https://doi.org/10.1074/jbc.m702615200 22. Heutinck KM, Berge TMJI, Hack EC, Hamann J and Rowshani AT. Serine proteases of the human immune system in health and disease (2010) Mol Immunol 47: 1943-1955. https://doi.org/10.1016/j.molimm.2010.04.020 23. Antonioli L, Fornai M, Pellegrini C and Blandizzi C. NKG2A and COVID-19: another brick in the wall (2020) Cell Mol Immunol 17: 672-674. https://doi.org/10.1038/s41423-020-0450-7 24. Hashizume M, Higuchi Y, Uchiyama Y and Mihara M. Il-6 plays an essential role in neutrophilia under inflammation (2011) cytokine 54: 92-99. https://doi.org/10.1016/j.cyto.2011.01.007 25. Wozniak A, Betts WH, Murphy GA and Rokicinski M. Interleukin-8 primes human neutrophils for enhanced superoxide anion production (1993) Immunology 79: 608-615. 26. Nandi B and Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection (2011) J Exp Med 208: 2251-2262. https://doi.org/10.1084/jem.20110919 27. Xu Z, Shi L, Wang Y, Zhang J, Huang L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome (2020) Lancet Respir Med 8: 420-422. https://doi.org/10.1016/S2213-2600(20)30076-X 28. Mason RJ. Pathogenesis of COVID-19 from a cell biologic perspective (2020) Eur Respir J. https://doi.org/10.1183/13993003.00607-2020 29. Magro C, Mulvey JJ , Berlin D , Nuovo G , Salvatore S, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases (2020) Translat Res 220: 1-13. https://doi.org/10.1016/j.trsl.2020.04.007 30. Zhou H, Deng M, Liu Y, Yang C, Hoffmann R, et al. Platelet HMGB1 is required for efficient bacterial clearance in intra-abdominal bacterial sepsis in mice (2018) Blood Adv. 2: 638-648. https://doi.org/10.1182/bloodadvances.2017011817 31. Conti P, Ronconi G, Caraffa A, Gallenga C, Ross R, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies (2020) J Biol Regul Homeost Age 34: 327-331. https://doi.org/10.23812/CONTI-E 32. Zhang C, Wu Z, Wen Li J, Zhao H and Qiang WG. The Cytokine Release Syndrome (CRS) of severe COVID-19 and Interleukin6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality (2020) Inter J Antimicrob Age 55. https://doi.org/10.1016/j.ijantimicag.2020.105954 33. Rizo VD, MartínezGuzmán MA, Gutierrez LI, Orozco AG, Navarro AA, et al. Neutrophil extracellular traps and its implications in inflammation: an overview (2017) Front Immunol 8. https://doi.org/10.3389/fimmu.2017.00081 34. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, et al. Neutrophil extracellular traps in COVID-19 (2020) JCI Insight 5. https://doi.org/10.1172/jci.insight.138999 35. Barnes BJ, Adrover JM, Stoltzfus AB, Borczuk A, Lartigue JC, et al. Targeting potential drivers of covid-19: neutrophil extracellular traps (2020) J Exp Med 217: e20200652. https://doi.org/10.1084/jem.20200652 36. Bruhl MLV, Stark K, Steinhart A, Chandraratne S, Konrad I, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo (2012) J Exp Med 209: 819-835. https://doi.org/10.1084/jem.20112322 37. Varatharajah N and Rajah S. Microthrombotic complications of covid-19 are likely due to embolism of circulating endothelial derived ultra-large von willebrand factor (eulvwf) decorated-platelet strings (2020) Fed Pract 37: 258-259. https://doi.org/10.12788//fp.0001 38. Garland KS, Reitsma SE, Shirai T, Rudenko JZ, Tucker EI, et al. Removal of the c-terminal domains of adamts13 by activated coagulation factor xi induces platelet adhesion on endothelial cells under flow conditions (2017) Front Med 4: 232. https://doi.org/10.3389/fmed.2017.00232 39. Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases (2010) Nat Med 16: 887-896. https://doi.org/10.1038/nm.2184 40. Chang JC. TTP-like syndrome: novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease (2018) Thrombosis Journal 16. https://doi.org/10.1186/s12959-018-0174-4 41. Lehman HK and Segal BH. The role of neutrophils in host defense and disease (2020) J Allergy Clin Immunol 145: 1535-1544. https://doi.org/10.1016/j.jaci.2020.02.038 42. Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, et al. Platelets adhered to endothelial cell-bound ultra-large von willebrand factor strings support leukocyte tethering and rolling under high shear stress (2005) J Thromb Haemost 3: 562‐570. https://doi.org/10.1111/j.1538-7836.2005.01122.x 43. Greene CM and McElvaney NG. Proteases and antiproteases in chronic neutrophilic lung disease-relevance to drug discovery (2009) British J Pharmacology 158: 1048-1058. https://doi.org/10.1111/j.1476-5381.2009.00448.x 44. Cera DE. Serine Proteases (2009) IUBMB Life 61: 510-515. https://doi.org/10.1002/iub.186 45. Thun GA, Ferrarotti I, Imboden M, Rochat T, Gerbase M, et al. SERPINA1 PiZ and PiS Heterozygotes and Lung Function Decline in the SAPALDIA Cohort (2012) PLOS ONE 7:e42728. https://doi.org/10.1371/journal.pone.0042728 46. Zhou P, Li T, Jin J, Liu Y, Li B, et al. Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion (2019) The Lancet 53. https://doi.org/10.2139/ssrn.3496918 47. Berangere SJ, Coppo P and Veyradier A. Thrombotic thrombocytopenic purpura (2017) Blood 129:2836-2846. https://doi.org/10.1182/blood-2016-10-709857 48. Federici BA, Falanga A, Lattuada A, Rocco DN, Barbui T, et al. Proteolysis of von Willebrand factor is decreased in acute promyelocytic leukaemia by treatment with all‐trans‐retinoic acid (1996) BJH 92: 733-739. https://doi.org/10.1046/j.1365-2141.1996.401939.x 49. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, et al. Convalescent plasma transfusion for the treatment of COVID‐19: Systematic review (2020) J Med Virol 92: 1475-1483. https://doi.org/10.1002/jmv.25961 50. Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews (2020). https://doi.org/10.1002/14651858.CD013600.pub2 51. Simonovich AV, Leandro D, Burgos P, Pratx P, Scibona P, et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia (2020) NEJM. https://doi.org/10.1056/nejmoa2031304 52. Benjamin RJ and McLaughlin LS. Plasma components: Properties, differences, and uses (2012) Transfusion 52: 9S-19S. https://doi.org/10.1111/j.1537-2995.2012.03622.x 53. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19 (2020) NEJM 382:e38. https://doi.org/10.1056/NEJMc2007575 54. Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis (2018) MBio. https://doi.org/10.1128/mbio.01753-18 55. Bosteels C, Neyt K, Vanheerswynghels M, Helden VJM, Sichien D, et al. Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection (2020) Immunity 52: 1039-1056. https://doi.org/10.1016/j.immuni.2020.04.005 56. Zhang L, Lin D, Sun X, Curth U, Drosten C, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors (2020) Science 368: 409-412. https://doi.org/10.1126/science.abb3405 57. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, et al. Structure, function, and antigenicity of the SARSCoV-2 spike glycoprotein (2020) Cell 183: 281-292. https://doi.org/10.1016/j.cell.2020.11.032 Corresponding author Neutrophilia, COVID-19, Proteolytic storm, SARS-CoV-2
infection.SARS-CoV-2 Infection is Protease-Dependent and Induces Neutrophils “Proteolytic Storm” Triggering Clinical Worsening and Viral Sepsis. Proteolysis and Inhibitors of Neutrophil Release Can Prevent and Treat Covid-19
Abstract
Full-Text
Introduction
Severe
Covid-19: Profound Immunologic Imbalance
Discussion
Conclusion
References
Citation
Fornasari MP. SARS-CoV-2 infection is protease-dependent and induces neutrophils
“proteolytic storm” triggering clinical worsening and viral sepsis. Proteolysis
and inhibitors of neutrophil release can prevent and treat covid-19 (2020) Edelweiss Appli Sci Tech 4: 67-73. Keywords