Review Article :
Anđelko Korušić, Viktor Đuzel, Vjekoslav Jeleč, Igor Nikolić, Miroslav Župčić and Duško Jovičić Percutaneous
tracheostomies, of any
technique, have become
an essential procedure in the ICU
setting, especially in patients for which we expect a need for prolonged
invasive ventilatory support. Percutaneous tracheostomies are very efficient, relatively
easily performed and thus need to be an essential skill for every intensivist. Experience
in setting an indication and timing is as equally important as the manual skill required
for the procedure.
Based on our
experience of over
300 performed percutaneous tracheostomies,
we recommend performing the procedure at an earlier phase, up to seven days
from intubation, in order to reduce the risks associated with prolonged
intubation such as tracheal stenosis, tracheo-oesophageal fistula formation and
ventilator associated pneumonia.As
with every invasive
procedure, there are
advantages and complications. Implementing a standardized
protocol for this procedure increases the rate of success and safety of the
patient. Based on available data, percutaneous tracheostomies have certain
definite advantage points in comparison to a classical surgical tracheostomy
and should therefore be recommended as a safe method of treatment, having in
mind that a classical surgical tracheotomy has its indications which must not
be overlooked. Tracheostomy is among the
oldest surgical procedures and has been known for over 5000 years. It has been noted
on Egyptian stone tablets which are dated some time around 3600 BC. It has also
been mentioned in ancient Hindu canonical texts such as the Rigveda (2000 BC.) It
was also known to the ancient Greek and Roman physicians. Asclepiades from Bythinia
was probably the first known individual to have performed a tracheostomy in 100
BC. The first successful tracheostomy was described by the Italian physician
Antonio Musa Brasavola in 1546. Up until the 18th century it was occasionally
used but only after 1820. did it become a widely used and well recognized
surgical procedure. After the report by P. Bretonneau about successful treatment
of a laryngeal obstruction due to dyphtheria, tracheostomy
became more popular as a treatment option, however still as a last resort
measure. Surgical tracheostomy
as we know it today, was first described by C. Jackson in 1909 [1]. The
technique was somewhat changed in the 1940s during the poliomyelitis epidemic,
but it is still essentially the same as the technique in use today. Throughout
the period between 1500. and 1833. only 28 successful tracheostomies have been reported.
During this time period, the mortality rate from the procedure was 70%. Surgical
tracheostomy is the most common surgical procedure in the intensive care unit
(ICU), on patients who require prolonged mechanical ventilation. Percutaneous tracheostomy
(PT) was first described by Shelden et al. in 1957 [2]. In 1967, Toy and
Weinstein described the Seldinger approach. In 1985,
Ciaglia et al. presented the percutaneous dilational tracheostomy (PDT), a technique
which utilises a needle, guide wire and subsequent dilation of the entry tunnel
with several incrementally wider dilators [3]. In 1989, Schachner
et al. presented the Rapitrac, a dilational surgical instrument which uses a sharp
point to quickly access the trachea through a guidewire, hence forming an
opening for the tracheal cannula [4]. Next year
Griggs et al. presented a similar technique (GWDF, guide wire dilating forceps)
and a new Rapitrac which in contrast to the older one, did not have such a
sharp point, thereby reducing the likelihood of bleeding and severe trauma [5]. Percutaneous
tracheostomy is not indicated in an emergency setting [6]. It is most commonly a
bedside procedure in the ICU when the patient is under analgesia, sedation, occasionally
relaxation and strictly monitored [7]. Immediately prior to the procedure, the
patient is preoxygenated with 100% oxygen and this inspiratory concentration is
maintained throughout the procedure, until the cannula is firmly in place,
until we are sure that there is no airway obstruction and the blood oxygen
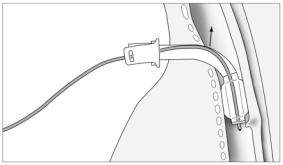
saturation is satisfactory. The neck is prepared in accordance with surgical
asepsis techniques as for any other surgical procedure. Note must be made of the
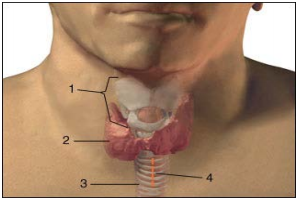
adequate size of the surgical field so that the individual performing the
procedure may comfortably and reliably palpate the landmarks (mm sternocleidomastoideus,
incisura jugularis, cartilago thyroidea, cartilago cricoidea, cartilago
trachealis) (Picture 1). The
puncture site is most commonly the fibromuscular part of the trachea, between the
second and third tracheal
cartilaginous rings. It is most easily located by moving the index finger from
the laryngeal prominence and in the mid line descending down to the second tracheal
cartilaginous ring. The laryngeal prominence is more emphasized in men (Adams apple)
and there is less subcutaneous fatty tissue in men. In any condition with thyroid
enlargement, when the thyroid isthmus is close to the puncture site, it is wiser
to perform a surgical tracheostomy in which the surgeon can visualise each layer
of tissue in advancing deeper towards the trachea. Even when the
initial puncture is uneventful, all of the subsequent procedures such as tissue
dilation with instruments, may cause a concealed haemorrhage (Picture 1). In the planned
site of puncture, a transversal incision of the skin and subdermis, of approximately
1.5-2 cm in length, is made. The skin and subdermal tissue contain small arteries
and veins which may bleed during the incision. Most commonly it will suffice to
gently compress the bleeding vessel and wait for the coagulation mechanism to
stop the bleed. If this does not suffice, sometimes it may be necessary to ligate
or cauterize the vessel. Just before incision, the area is usually anaesthetized
with 3-5 ml of 1% lidocaine with adrenaline (1:200000) to minimise the bleeding.
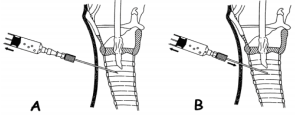
Puncture is performed using a 14G needle with a plastic cannula, which remains in
the trachea after the needle is pulled out. We must keep in mind that the trachea
is most often quite superficial and usually just 1 cm under the skin, so caution
must be exercised during puncture in order to avoid pushing the needle too deep
and puncturing the vessels beneath the trachea, such as the brachiocephalic trunk,
which may cause a potentially fatal haemorrhage. Anterior
and superficial to the trachea, sometimes there is thyroid tissue and it also
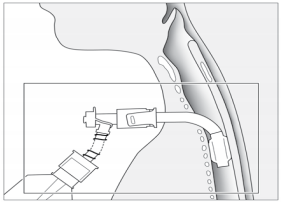
can bleed profusely. The direction of the needle is perpendicular to the skin. During
puncture the position of needle insertion depth is verified by aspiration of air
from the trachea into the syringe, which is usually filled with normal saline
(Picture 2). The appearance
of air in the syringe which is prefilled with normal saline, is an
indicator of correct position. The endotracheal tube, which was retracted
approximately 2-3 cm prior to incision, is gently rotated 30 degrees to either
side after puncture, to make sure that the needle did not puncture the endotracheal
tube. The aspiration test will not distinguish the lumen of the tube from that of
the trachea. Once the correct intratracheal position
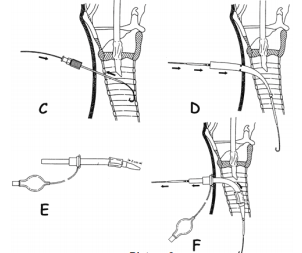
is confirmed, a guidewire is inserted through the plastic insertion cannula and
is left in place while the small insertion cannula is removed (Picture 3). Up to this
point, both of the most common techniques are essentially the same. Ciaglias technique
further recommends a blunt dissection of the tracheal aperture via a rigid plastic
dilator of a smaller diameter. After this dilation, a cone shaped dilator is inserted
over the guidewire and is inserted up to the marked line which indicates that the
aperture is wide enough for insertion of the cannula. A cone shaped catheter is
inserted through the cannula with its tip coming slightly out in front of the cannula
and this serves for easier passage of the cannula through the tracheal aperture.
The cannula with the catheter is inserted over the guidewire and once the cannula
is in the right position, the guidewire and catheter are removed from the cannula
and the cannula remains in place (Picture 3). Griggs technique
forms an initial stoma with the aid of a plastic dilator and the uses a special
speculum like forceps which is placed over the guidewire into the trachea and
is then widened causing a blunt spreading of the tracheal tissue and thus a widening
of the aperture (Pictures 4 and 5). Both techniques
may require significant physical force because the surrounding tissue may prove
to be quite resistant to blunt dissection. Once the tracheal aperture is assessed
as sufficiently wide and appropriate in shape, the tracheostomy cannula is inserted.
The cannula must be well lubricated with gel so it can easily bypass the tissue
around the aperture. A problem which may arise while passing the cannula through
the trachea is when the rigid tracheal cannula tip gets stuck or encounters the
tracheal rings en route. If the curved introducer is inserted in through the cannula,
it will curve the cannula and make its passage easier along the tracheal rings and further
through the trachea. Another problem which may potentially arise is the necessity
of extraction of the guidewire prior to cannula insertion and subsequent possibility
of paratracheal cannula insertion. In younger patients with a more firm trachea
and well formed aperture there are usually no significant difficulties in
inserting the cannula without the guidewire in situ, however, in older patients
sometimes it is almost impossible to insert the cannula without the guidewire. The
problem should be solved once the manufacturers make an additional opening for
the guidewire through the curved introducer which goes into the cannula, so the
guidewire will not have to be removed. When advancing the cannula through the trachea,
caution must be exercised in following the curvature of the wire which follows the
anatomical curvatures of the aperture and trachea. The guidewire may also be
contorted immediately below the tip of the cannula if the cannula tip is at a
steep angle in relation to the guidewire, that is why it is necessary to slightly
and gently move the guidewire in and out while advancing the cannula into the
trachea, to ensure it has not curved and has remained mobile. This same maneuvre
is recommended while performing tracheal aperture dilation with the forceps in
Griggs technique. After inserting
the tracheal cannula, the trachea should be suctioned and with an aspiration catheter.
The cuff should be inflated with attention to cuff pressure and ventilation may
commence via the cannula. The endotracheal tube is removed and hygiene of the
oral cavity and upper airways is performed. Cannula position and patency is verified
by auscultation, chest –xray and fiberoptic bronchoscopy. Fiberoptic
bronchoscopy before, during and after tracheal cannula insertion will give a complete
insight into the condition of the trachea and envisage any potential complications
(bleeding, tracheal wall
lacerations, tracheal cartilage trauma or rupture) in a timely manner. The time needed
to carry out a percutaneous tracheostomy has become important in assessing the success
of this technique in comparison to other techniques. The inability to adequately
dilate a traheal
aperture, increased number of attempted dilations (more than one attempt)
and prolonged cannula insertion time are indicators of complications and they
are indicative of categorizing a tracheostomy as insufficiently dilated. It is
necessary to mention the complication of tracheal cannula obstruction with consequential
desaturation of arterial oxygen which can occur a few days after successful percutaneous
tracheal cannulation (usually occurs between day 2 and 21 post tracheostomy).
Partial obstruction may occur due to the weight of the cannula itself and the
weight of the tubing connected to the respirator. When a patient is in the
supine position for a prolonged period of time, there is usually some degree of
lateral deviation of the cannula due to the continued traction by the hosing system
on the side of the respirator, while in seated patients who require mechanical ventilation
support, the cannula usually deviates downwards. The weight of the whole system
causes the cannula to deviate in position and the walls of the cannula are no
longer in alignment with the walls of the trachea Obstruction
occurs for two reasons. Tracheal wall trauma makes the wall of the trachea softer
and easy to deform so the deviation of the cannula and the traction on the tissue
causes mucosal folds to appear in front of the cannulas meatus thereby causing a
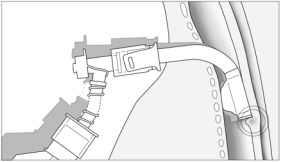
partial obstruction to the free flow of air. The same happens when the cuff of
the cannula deflates or loses its elasticity and movement of the cannula causes
a cuff herniation and
consequential partial obstruction of the airway (Picture 7). In scenarios
where there is a desaturation of arterial oxygen content it is always prudent
to perform a fiberoptic bronchoscopy however it is not always easy to ascertain
the problem due to the fact that when a fiberoptic bronchoscope is inserted,
the cannula is usually straightened and the obstructions may disappear in that moment
(Picture 8). The
problems and solutions were discovered empirically, while performing airway
suction and cleaning maneuvres. It was noticed that in certain positions of the
cannula there was an occurence of desaturation and return to normal values when
the cannula was repositioned (Picture 6). The advantages
of percutaneous tracheostomy need to be considered in comparison to classic surgical
tracheostomy. Outcomes and results of numerous studies which have been comparing
various techniques mostly depend on study designs and observed parameters. Even
though there are no definite and conclusive results meta analyses of studies
suggest that percutaneous tracheostomy is less time consuming, less traumatic, associated
with fewer intraoperative and postoperative complications and is more cost
effective than a classical surgical tracheostomy
[8]. Note must be made of the studies which conclude that percutaneous tracheostomy
showed more periprocedural complications, especially those studies that mention
fatalities and cardiac arrest. These meta analyses were made on the basis of all
percutaneous tracheostomies irrespective of the technique used [9]. Outcomes and
results also differ between the techniques of percutaneous tracheostomy. Griggs
GDWF method, is somewhat less time consuming whereas for the associated
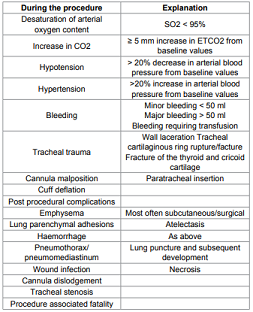
bleeding which is During the procedure Explanation Desaturation of arterial oxygen
content SO2 < 95% Increase in CO2 ≥ 5 mm increase in ETCO2 from baseline
values Hypotension > 20% decrease in arterial blood pressure from baseline
values Hypertension >20% increase in arterial blood pressure from baseline
values Bleeding Minor bleeding < 50 ml Major bleeding > 50 ml Bleeding
requiring transfusion Tracheal
trauma Wall laceration Tracheal cartilaginous ring rupture/facture Fracture
of the thyroid and cricoid cartilage Cannula malposition Paratracheal insertion
Cuff deflation Post procedural complications Emphysema Most often
subcutaneous/surgical Lung parenchymal adhesions Atelectasis Haemorrhage As
above Pneumothorax/ pneumomediastinum Lung puncture and subsequent development Wound
infection Necrosis Cannula dislodgement Tracheal stenosis Procedure associated
fatality Table 1: Complications. Apparently more incidental with GWDF and apparently
more pronounced hypotension there is no statistical confirmation [10]. Both techniques
are equally reliable when observing the overall outcome with a differential emphasis
on increased peak airway pressure with GDWF and tracheal ring fracture in
Ciaglias technique, however without statistically significant difference. Most
studies failed to find significant differences between the two techniques [11].
All comparisons,
between classical surgical and percutaneous tracheostomies and between percutaneous
tracheostomy techniques, put a significant emphasis on the skills of the
operator carrying out the procedure [12]. There was a significant reduction in
the rate and severity of complications if the operator had more experience with
15 or more successful previous tracheostomies. In the Clinical
department of Anesthesia, Reanimation and Intensive Care Medicine
at University Hospital Dubrava, we have performed 250 percutaneous tracheostomies
through 10 years. We have applied both of the mentioned techniques and have
even combined the two techniques to make a third separate technique. Among the complications
were ten minor bleeds, three were converted ultimately to a classical surgical tracheostomy
due to bleeding from the thyroid and a few of the patients experienced an
increase in arterial blood pressure during the procedure. There were no instances
of oxygen desaturation in any of the cases. Four of the patients died a month after
the procedure with the tracheostomy cannula in situ. These were one neurosurgical, one maxillofacial
and two cardiosurgical
patients respectively. We consider
that tracheostomies are a vital element of the intensive care treatment armamentarium
irrespective of the technique used. They are recommendable as treatment options
for patients who require prolonged mechanical ventilation. They are very effective,
easily performed and should be a required skill for all intensive care physicians.
Experience in indicating the procedure and appropriate timing are equally as important
as the manual skill in performing the procedure. We recommend an early consideration
for the procedure, preferably within a week if there is no anticipated likelihood
of weaning from mechanical ventilation and extubation, in order to reduce the
risks associated with prolonged intubation. As with every invasive procedure, percutaneous
tracheostomies have their advantages and complications. Based on the
discussion in this review, especially considering the advantages in comparison
to a classical surgical tracheostomy, we can recommend percutaneous tracheostomy
as a safe and effective method of treatment, however with a note that a classical
surgical tracheostomy
still has its indications and should always be considered with regard for the safest
treatment option for the patient. 1. Jackson
C. Tracheostomy. (1909) Laryngoscope 19:285-290. Anđelko Korušić, Department of Anaesthesiology,
Reanimatology and Intensive Care, University Hospital Dubrava, Av. Gojka Šuška
6, 10000 Zagreb, Croatia, Tel: 385 1 2902797/+385 98 358262; Fax: 385 1 2902793
E-mail: akorusic@gmail.com Korušić A, Đuzel V, Jeleč V, Nikolić I, Župčić M, et al.
(2016) Percutaneous Tracheostomy – Advantages and Complications. NHC 103: 14-18 Percutaneus Tracheostomy
Percutaneous Tracheostomy – Advantages and Complications
Abstract
Full-Text
History
Procedure


Complications
of the Procedure
Advantages
of Percutaneous Tracheostomy
Conclusion
References
2. Shelden CH, Pudenz RH, Tichy FY. Percutaneous tracheotomy. (1957) J Am Med
Assoc 165: 2068-2070.
3. Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy.
A new simple bedside procedure; preliminary report. (1985) Chest 87:715-719.
4. Schachner A, Ovil J, Sidi J, Avram A, Levy MJ. Rapid percutaneous tracheostomy
(1990) Chest 98: 1266-1270.
5. Griggs WM, Worthley LI, Gilligan JE, Thomas PD, Myburg JA. A simple percutaneous
tracheostomy technique. (1990) Surg Gynecol Obstet 170: 543-545.
6. Ciaglia P. Improving percutaneous dilational tracheostomy. (1997) Chest 112:
295.
7. Fantoni A, Ripamonti D. A non-derivative, non-surgical tracheostomy: the
translaryngeal method. (1997) Intensive Care Med 23: 386-392.
8. Polderman KH, Spijkstra JJ, Remco de Bree, Christiaans HMT, Gelissen HPMM,
et al. Percutaneous Dilatational Tracheostomy in the ICU* Optimal Organization,
Low Complication Rates, and Description of a New Complication. (2003) Chest.
123: 1595-1602.
9. Dob DP, McLure HA, Soni N. Failed intubation and emergency percutaneous
tracheostomy. (1998) Anaesthesia 53: 72-74.
10. Añón JM, Gómez V, Escuela MP, De Paz V, Solana LF, et al.. Percutaneous
tracheostomy: comparison of Ciaglia and Griggs techniques. (2000) Crit Care 4:
124-128.
11. Basaranoglu G, Erden V. Failed intubation due to posterior fossa haematoma requiring
emergency percutaneous tracheostomy (2002) Br J Anaesth 88: 310-311.
12. American Society of Anesthesiologists Task Force on Management of the Difficult
Airway. Practice guidelines for management of the difficult airway: an updated
report by the American Society of Anesthesiologists Task Force on Management of
the Difficult Airway. (2003) Anesthesiology 98: 1269-1277. *Corresponding author
Citation
Keywords