Review Article :
Our planets community
largely depends on a snug energy supply, and non-renewable energy such as
fossil fuel has been serving as the most trustworthy energy source from its
discovery time of 1673 till to the current century. However, non-renewable
energy resources are rapidly decreased per year due to increasing the energy
consumption rate. To address this issue, renewable energy chiefly photovoltaic
energy has attracted much though, because it directly converts solar energy
into electrical without environment pollution. For the past several years,
different photovoltaic devices like inorganic organic, and hybrid solar cells
are invented for different application purposes. Regardless of its high
conversion rate of silicon based solar cells, the high module cost and complicated
production process restricted their application. Research has been focused on
alternative organic solar cells for their inherent low module cost and easy
fabrication processes. From all organic solar cells, Dye-Sensitized Solar Cells
(DSSCs) are the most efficient, low cost and easily implemented technology.
This review paper focus on clarifying the technological meaning of DSSCs, Types
of DSSCs materials, working principle, advantages, power full applications area
of DSSCs, the efficiency and challenges for R&D of DSSCs to upgrade the
current efficiency. The
consumption of global energy is increasing year by year. As the research
progress show, in 1998, it was 12.7 TW, but in 2050, it is expected to be
around 26.4 to 32.9 TW and in 2100, it will increase up to 46.3 to 58.7 TW
[13]. The solar
radiation from the sun is approximately 3×1024 J per year, which
are ten times the current energy demands of the world [21,65]. As
the storage of a fossil supply is ebbing every year the mankind must look for
another source of energy [11,18,65]. The sun is a primary source of energy for
most life forms in our planet. It is clear, abundant and renewable [14,65]. By
fully grasping the power of the sun we can improve our way of life, reduce our
dependence on fossil fuels or other types of energy sources and stimulate
economy by bringing new jobs to all our planet industry. Among
sustainable and renewable energy resources, such as tidal power, solar thermal,
hydropower and biomass, solar cell which is also known as photovoltaic cell is
one of the promising options of renewable energy and the most efficient
[22,23]. Among different categories of solar cell, the dye-sensitized
solar cells (DSSC), which is invented by Professor M. Grätzel in 1991 (ORegan
& Grätzel, 1991) [18,26], is a most promising inexpensive route toward
sunlight harvesting. DSSCs are belong to the thin film group, emerged as a new
class of low cost energy conversion devices with simple manufacturing
Procedures [13]. The good light-harvesting efficiency of the best desensitized
solar cells (DSSCs) is the product of a dye
with moderate extinction and a photo anode of high surface area (∼1200
times the area of a flat electrode).This combination allows for ample
absorbance over the majority of the visible spectrum with room for improvement
in the red wavelengths [8,15,17]. The
fundamental component of the DSC is a photo anode consisting of a monolayer
of sensitizer (dye) adsorbed onto a mesoporous semiconductor oxide
(typically TiO2). In contrast to conventional solar cell systems,
where the semiconductor assumes both the task of light absorption and charge
carrier, in dye-sensitized solar cells light is absorbed by the anchored dye
and charge separation takes place at the interface via photo induced electron
injection from the dye into the conduction band of the solid [7,61]. In
general highly efficient photovoltaic conversions, combined with ease of
manufacturing and low production costs [6], make the DSC technology an
attractive approach for large-scale solar energy conversion comparing to other
forms of solar cell. In
this review paper, the general DSSCs benefits and application, DSSCs materials,
working principles, efficiency increment due to new materials investigation
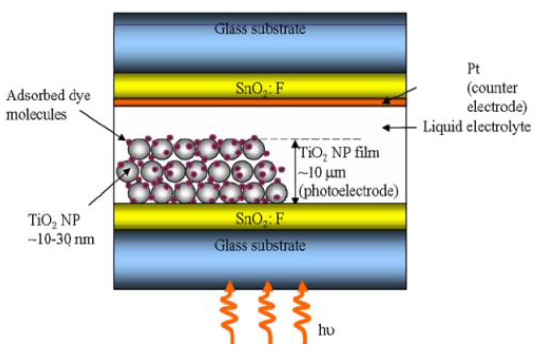
that suit for DSSC and research challenges will be discussed. The current DSSC construction
involves a set of different layers of components, including glass substrate,
transparent conducting layer,TiO2 nanoparticles, dyes, electrolyte
(I-/ I-3 or Co II / Co III complexes), and
counter electrode (Carbon or Pt) covered with sealing gasket. The typical
construction of DSSC is shown in Figure
1. The main in
dye-sensitized solar cells components, including semiconductor films, dye
sensitizers nonporous, redox electrolyte, conducting substrate and counter
electrode [52]. Figure 1: Typical design of a dye-sensitized
solar cell Transparent and
Conductive Substrate DSSCs are typically
constructed with two sheets of conductive transparent materials, which help a
substrate for the deposition of the semiconductor and catalyst, acting also as
current collectors [66]. Substrates necessarily are high transparent
(transparency > 80%) to permit the passage of optimum sunlight to the
effective area of the cell. Its electrical conductivity should also be high for
efficient charge transfer and to decrease energy loss. These two
characteristics of substrate dictate the efficiency of DSSCs [21,66]. Typically, FTO (fluorine
tin oxide, SnO2: F) and ITO (indium tin oxide, In2O3:
Sn) are used as the conductive substrate. ITO and FTO and ITO substrates
consist of soda lime glass coated with indium tin oxide layers and fluorine tin
oxide, respectively. ITO films have a transmittance of above 80% and sheet
resistance of 18 Ω/cm2, while FTO films show a transmittance of
about 75% in the visible region and sheet resistance of 8.5/cm2
[21]. Nano Crystalline Semiconductor film Electrode Semiconductor oxides
used in dye-sensitized solar cell include SnO2, Nb2O5,
TiO2, ZnO, and so forth, which serve as the carrier for the
monolayers of the sensitizer using their high surface and the medium of
electron transfer to the conducting substrate. Due to low-cost price, abundance
in the market, nontoxicity, and biocompatibility, and as it is also used widely
in health care products as well as in paints, TiO2 becomes the best
choice in semiconductor till now [52]. Titanium
dioxide (TiO2) films are covered on the conducting substrate
such as metal foil, flexible polymer film and conducting glass. Dye sensitizers serve
as the solar energy absorber in DSC, whose proprieties will have much effect on
the light
harvesting efficiency and the overall photoelectric
conversion efficiency. The ideal sensitizer for dye-sensitized solar cells
should absorb all light just below a threshold wavelength of 920 nm and firmly
grafted to the semiconductor oxide surface and inject electrons to the
conduction band with a quantum yield of unity [11,52].Its redox potential
should be sufficiently high that it can be regenerated rapidly via electron
donation from the electrolyte or a hole conductor. Finally, it should be stable
enough to sustain at least 108 redox turnovers under illumination corresponding
to about 20 years of exposure to natural light [19]. The purpose of dye is
to absorb light and exchange electrons to the conduction band of the
semiconductor. It is chemically bonded to the porous surface of the semiconductor.
An efficient photosensitizer should [21, 66]: 1. Show excellent absorption in the visible region (400nm to
700nm), 2. Adsorb strongly on the surface of the semiconductor, 3. Has a high extinction coefficient, 4. Be stable in its oxidized form allowing it to be reduced by an
electrolyte, 5. Be stable enough to carry out ∼108 turnovers, which
typically correspond to 20 years of cell operation, 6. Possess more negative LUMO than the CB of the semiconductor and
more positive HOMO than the redox potential of the electrolyte. In general there are
three classes of photosensitizers: metal-free organic sensitizers, natural
sensitizers and metal complex sensitizers [66]. Metal complex sensitizers comprise of both Anchoring
Ligands (ACLs) Ancillary
Ligands (ALLs). The adhesion of photosensitizers to the semiconductor is
highly dependent on the properties of ACLs. While ALLs can be used for the
tuning of the overall nature of sensitizers, polypyridine complexes of d6 metal
ions possess very high Metal
To Ligand Charge Transfer (MLCT) bands in the visible region which is shown
by polypyridine complexes of d6 metal ions [21]. Metal - Free Photo Sensitizers Metal free organic
sensitizers have been used both to replace the expensive ruthenium based
sensitizers and to improve the electronic properties of devices. Even though,
the efficacy of these sensitizers is still low when compared to devices based
on ruthenium-based dyes, the efficacy and performance can be improved by the
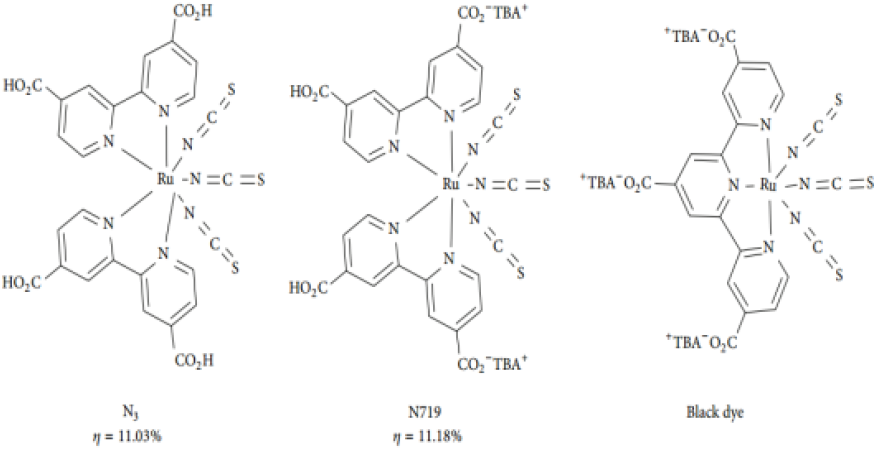
proper selection or tuning of the designing components. Natural Sensitizers Natural dyes have
also been used in DSSCs due to their low cost, easy extraction, nontoxicity,
and the environmentally benign nature [22] (Figure 2). Figure 2: Structure of some efficient Ru-based
photosensitizers adapted from Electrolyte The purpose of the electrolyte is
to regenerate the dye after it injects electrons into the conduction band of
the semiconductor. It also acts as a charge passage medium to transfer positive
charges toward the counter electrodes. The long-functional life time stability
of DSSCs strongly depends on the properties of electrolyte. Thus, the
electrolyte must have the following characteristic [21,19]. 1.
Excellent electrical conductivity
and low viscosity for faster diffusion of electrons. 2.
Good interfacial contact with the
nanocrystalline semiconductor and the counter electrode. 3.
It should not be the cause of
desorption of the dye from the oxidized surface and the degradation of the dye. 4.
It should not absorb light in the
visible region. Electrolytes
for DSSCs are classified into three types: solid state electrolytes, liquid
electrolytes, and quasi solid state electrolytes. Liquid
electrolytes are basically classified into two types: organic solvent based
electrolytes and room temperature ionic liquid electrolytes (RTIL) based on the
solvent used. Organic Electrolytes: Each component of
organic electrolytes such as the redox couple, solvent, and additives affects
the performance of DSSCs. The major component of organic electrolyte is the
redox couple. Many types of redox couples such as Br−/Br3, SCN−/
(SCN)2, SeCN−/(SeCN)2 [21,24], and substituted bipyridyl
cobalt (III/II) [52] have been investigated. But I3−/I− is considered an ideal
redox couple because of its excellent solubility, rapid dye regeneration, low
absorbance of light in the visible region, suitable redox potential, and very
slow recombination kinetics between injected electrons into the semiconductor
and triiodide [13]. Ionic
Electrolytes:
RTIL have been employed successfully for reduction of a high evaporation rate
due to high volatility of liquid electrolytes. They are a group of organic
salts containing captions such as pyridinium, imidazolium, and anions from the
halide or pseudohalide family [19]. They act simultaneously as an iodine source
and as a solvent. Solid-State
Electrolyte Leakage
is the main problem in liquid-electrolyte based DSSCs, which drastically
minimize the long-term stability of solar cells. In order to upgrade the
performance and stability, solid state electrolytes have been developed. They
replace the liquid electrolyte with a p-type semi-conductor [21]. Counter
Electrode The
counter
electrode is used for the regeneration of the electrolyte. The oxidized
electrolyte diffuses towards the counter electrode where it receive electrons
from the external circuit. A catalyst is needed to accelerate the reduction
reaction and platinum (Pt) is considered a preferred catalyst due to its high
exchange current density, good catalytic activity, and transparency. The
performance of the CE depends on the method of Pt deposition on TCO substrate
[19, 22]. Working
Principles of DSSC The basic operational principles
of DSSC solar cells in comparison with conventional semiconductor solar cells
are different. In semiconductor solar cells light absorption and charge carrier
transport are not the separate task. In DSSC these two tasks are separate.
Charge separation is done by photo-induced injection to the conduction band and
such created carriers are transported to charge collector [11]. By using dyes
the solar cell is capable to harvest large fraction of sunlight due to its high broad
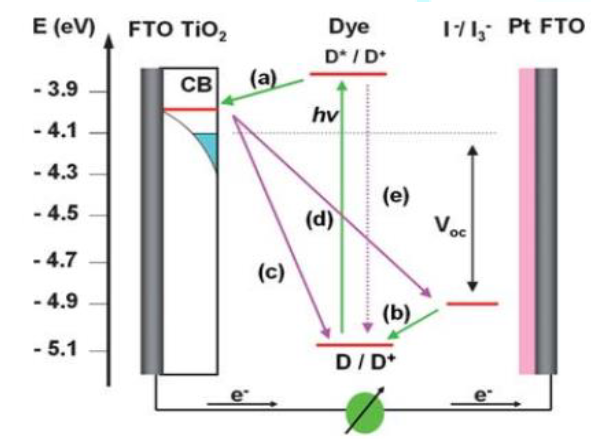
absorption band. Figure 3 shows the energy band structure of the DSSC device
and principal carrier transfer channels [1] There are many other undesirable
carrier transfer channels including charge recombination of the injected
electrons from the TiO2 CB (defined as the injected electron) to
cations of the dyes (c) and to redox couples (d), and direct decay from the
LUMO to the HOMO of the dye (e). Where
D represents dye sensitizer. In principle, the energy
conversion efficiency of a DSSC is the product of the short-circuit
photocurrent, Jsc, the open-circuit photovoltage Voc, as
well as the fill factor [1, 11]. Referring to the energy band structure
and the carrier transfer processes in Fig. 1, the Voc is calculated
by the following equation. Where n is the number of the
electrons in TiO2, NCB is the effective density of states
at conduction band, Eredox is the HOMO level of the redox couples,
and q is the unit charge in coulomb. Efficiency of
DSSCs Tremendous research efforts have
been invested to improve the efficiency of solar energy conversion which is
generally determined by the light harvesting efficiency, electron injection
efficiency and undesirable charge recombination degree. Pursuing high
efficiency is always the core task for photovoltaic devices. For DSSC, overall
energy conversion efficiency (η) of 11.0% has been achieved at AM 1.5 [7]
.In order to further enhance the energy conversion efficiency of DSSCs, it is
critical to improve the Voc by (1) reduce the charge recombination
between redox couple and the injected electrons in the TiO2 CB; (2)
reduce charge recombination between the oxidized sensitizer and the injected
electrons in the TiO2 CB; (3) increase the electron injection
efficiency; (4) increase the TiO2 ECB; (5) downshift the Eredox; (6)
tandem DSCs.[1]. To reduce charge recombination, the following factors about
sensitizers should be considered. First, it should form a compact blocking
layer on the TiO2 surface. Secondly, the undesirable complexation
between the sensitizer and iodide should be prevented. Thirdly, the electron
donor unit should be separated from the TiO2 surface to impede
charge recombination between the injected electrons and the oxidized
sensitizer. To improve electron injection efficiency, molecular aggregation
should be prevented and the LUMO of the sensitizer should overlap well with
that of TiO2. Finally, to broaden the absorption spectra of the
sensitizer, strong electron donor and acceptor groups might be a good choice.
Furthermore, multiple electron donor substituents are encouraged under the
condition that the oxidized sensitizer can be reduced effectively by the redox
couple. The fast-developing organic sensitizers are promising for reinforcing
the Voc and efficiency by exquisite molecular tailoring. Where Voc; open circuit
voltage, Isc; short circuit current. Imax and Vmax
are the maximum cell current and voltage respectively at the maximum power
point, Pmax = Imax x Vmax . The incident photon conversion
efficiency (IPCE) of DSSC is an incident energy-dependent quality. It is a
measure of the useful range of the cell. The IPCE is given by Where λ is wavelength, PINincident optical
power, e is the fundamental electron charge, h Plancks constant
and c is the speed of light in vacuum. The global power conversion
efficiency of energy to electricity conversion efficiency (η) of a cell with
Pout electrical power under standard illumination conditions is given by As the different researcher
progress work on DSSC shows, it has currently have low conversion efficiency.
Many researchers have attempted to resolve this problem, by increasing the
surface area of TiO2 photo-electrodes used in the DSSC [62]. Low efficiency and low stability
are the major challenges for the commercial deployment of DSSCs [21].The main
causes of low efficiency in DSSCs are 1.
Low red and near-IR absorption. 2.
Low extinction coefficient
requires high surface area. 3.
Only redox couple has slow
recombination kinetics, but it has unnecessarily large over potential. 4.
Poor contact between the
electrodes 5.
Degradation of electrolyte
properties due to UV absorption of light. Improving the environmental
stability of cells is the most important issue in studying these cells [60]. Stability
refers to the performance of individual processes or the entire solar cell at
any time relative to the initial time. Good stability leads to long lifetimes
[59]. The critical issue regarding to stability and robustness of DSSCs are 1.
Liquid electrolyte is undesirable,
but solid state hole conductors give lower efficiency. 2.
Achieving DSSC module lifetimes of
more than 20 years requires 108 turnovers for dye molecules and high
quality encapsulation to prevent leakage of the electrolyte and ingress of water
[59]. 3.
Is corrosive. Dye-sensitized solar cells have
the following main advantages: Colorable,
transparent: The
use of dye allows wide selection of colored cells and transparent cells. The
transparency and varied color of DSSCs could be utilized for decorative
purposes like window and sunroof [58]. Flexible and thin
structure: By
using aggregates of fine particles of photoelectric conversion materials, the
solar cells can be formed as flexible thin films. Generation
characteristics of insusceptible to the incident angle and intensity of the
sunlight: Even
though the light condition is very week generation characteristics can be maintained,
such as under faint light in the morning and evening and when indoors. Environmentally
friend and recyclable Dye-sensitized solar cells do not
have harmful substance as a cell component material. The materials are comparatively
easy to separate and get back, which is advantageous in view
of a recycling and reuse framework for solar cell panels [63]. In conclusion, the worlds nonrenewable energy
degrades time by time and the consumption rate increases inversely. To weaken
these two controversies, new environmental friend green renewable energy
resources are highly needed to our planet. Among different types of renewable
green energy resources, solar energy is regarded as one of the perfect energy
resources. There has been a continuous effort
in searching for affordable organic solar energies among which dye-sensitized
solar cells (DSCs) thus far demonstrate the highest energy conversion
efficiency, and have been regarded as the most prospective technology in the
near future. Dye-sensitized solar cells have gained widespread attention in
recent years because of their low production costs, easy of fabrication, its
lighter weight property, environmentally friend and recyclable advantages and
tunable optical properties, such as color and transparency regardless of its
low efficiency output comparing to silicon solar cell. Figure 4: Prototype Models of Dye-Sensitized
Solar Cell Panels for decoration purpose 1.
Zhijun Ning, Ying Fu and He Tian.
Improvement of dye-sensitized solar cells: What we know and what we need to
know (2010) Energy Environmental Science. DOI: 10.1039/C003841E 2.
Jason B Baxter and Eray S Aydil.
Nanowire-based dye-sensitized solar cells (2005) Applied Physics Letters 86:
053114. https://doi.org/10.1063/1.1861510 3.
Renu Guliani, Amit Jain and
Avinashi Kapoor. Exact Analytical Analysis of Dye-Sensitized Solar Cell:
Improved Method and Comparative Study (2012) The Open Renewable Energy J 5:
49-60. http://dx.doi.org/10.2174/1876387101205010049 4.
QB Meng, K Takahashi, XT Zhang, I
Sutanto, TN Rao, et al. Fabrication of an Efficient Solid-State Dye-Sensitized
Solar Cell (2003) Langmuir, 19: 3572-3574. DOI: 10.1021/la026832n 5.
Jeong-Hyeok IM, Chang-Ryul Lee,
Jin-Wook Lee, SangWon Park and Nam-Gyu Park. 6.5% efficient perovskite
quantum-dot-sensitized solar cell (2011) Nanoscale 3: 4088. DOI:
10.1039/C1NR10867K 6.
Seigo Ito, Takurou N. Murakami,
Pascal Comte, Paul Liska, Carole Grätzel, et al. Fabrication of thin film dye
sensitized solar cells with solar to electric power conversion efficiency over
10% (2008) Elsevier Thin Solid Films 516: 4613-4619. https://doi.org/10.1016/j.tsf.2007.05.090 7.
Qing Wang, Seigo Ito, Michael
Gra1tzel, Francisco Fabregat-Santiago, Iva´n Mora-Sero´, et al. Characteristics
of High Efficiency Dye-Sensitized Solar Cells (2006) J Phys Chem B 110:
25210-25221. https://doi.org/10.1021/jp064256o 8.
Alex BF Martinson, Jeffrey W
Elam, Joseph T Hupp and Michael J Pellin. ZnO Nanotube Based Dye-Sensitized
Solar Cells (2007) Nano letters 7: 2183-2187. DOI: 10.1021/nl070160+ 9.
Jason B Baxter. Dye Sensitized
Solar Cells: R&D Issues (2010) NSF PV Workshop. 10.
Simon Mathew, Aswani Yella, Peng
Gao, Robin Humphry-Baker, Basile FE Curchod, et al. Dye-sensitized solar cells
with 13% efficiency achieved through the molecular engineering of porphyrin
sensitizers (1861) Nature chemistry. https://doi.org/10.1038/nchem.1861 11.
Michal Sokolský and Július Cirák.
Dye-sensitized solar cells: Materials and processes (2010) Acta Electro
technica et Informatica 10: 78-81. 12.
Rajaram S. Mane, Won Joo Lee,
Habib M. Pathan and Sung-Hwan Han. Nanocrystalline TiO2/ZnO Thin
Films: Fabrication and Application to Dye-Sensitized Solar Cells (2008)
Physical chemistry 109: 24254–24259. DOI: 10.1021/jp0531560 13.
Arini Nuran Binti Zulkifili,
Terauchi Kento, Matsutake Daiki and Akira Fujiki. The Basic Research on the
Dye-Sensitized Solar Cells (DSSC) (2015) J Clean Energy Technologies 3. DOI:
10.7763/JOCET.2015.V3.228 14.
Andigoni Apostolopoulou, Dimitris
Karageorgopoulos, Andreas Rapsomanikis and Elias Stathatos. Dye-Sensitized
Solar Cells with Zinc Oxide Nanostructured Films Made with Amine Oligomers as
Organic Templates and Gel Electrolytes (2016) J Clean Energy Technologies. DOI:
10.18178/JOCET.2016.4.5.303 15.
Takeru Bessho, Shaik M
Zakeeruddin, Chen-Yu Yeh, Eric Wei-Guang Diau and Michael Grtzel. Highly
Efficient Mesoscopic Dye-Sensitized Solar Cells Based on
Donor–Acceptor-Substituted Porphyrins (2010) Communications. https://doi.org/10.1002/anie.201002118 16.
Yong-Bing Tang, Chun-Sing Lee,
Jun Xu, Zeng-Tao Liu, Zhen-Hua Chen, et al. Incorporation of Graphenes in
Nanostructured TiO2 Films via Molecular Grafting for Dye-Sensitized
Solar Cell Application. DOI: 10.1021/nn100449w 17.
Zhen Huang, Xizhe Liu, Kexin Li,
Dongmei Li, Yanhong Luo, et al. Application of carbon materials as counter
electrodes of dye-sensitized solar cells (2007) Elsevier, Electrochemistry
Communications 9: 596-598. https://doi.org/10.1016/j.elecom.2006.10.028 18.
Yang Jiao, Fan Zhang and Sheng
Meng. Dye Sensitized Solar Cells Principles and New Design. Beijing China. 19.
Khalil Ebrahim Jasim. Dye Sensitized
Solar Cells - Working Principles, Challenges and Opportunities. Department of
Physics, University of Bahrain Kingdom of Bahrain. 20.
Geargg. A low cost, high
efficiency solar cell based on dye synthesized colloidal Tio2 films
(2015) Nature. https://doi.org/10.1038/353737a0 21.
Umer Mehmood, Saleem-ur Rahman,
Khalil Harrabi, Ibnelwaleed A. Hussein, BVS. Reddy. Recent Advances in Dye
Sensitized Solar Cells (2014). 22.
Suriati Suhaimi, Mukhzeer Mohamad
Shahimin, ZA Alahmed, J Chyský and AH Reshak. Materials for Enhanced
Dye-sensitized Solar Cell Performance: Electrochemical Application (2015) Int J
Electrochem Sci 10: 2859-2871. 23.
Sanghoon Yoon, Sehyun Tak, Jinsoo
Kim, Yongseok Jun, Kisuk Kang, et al. Application of transparent dye-sensitized
solar cells to building integrated photovoltaic systems (2011) Elseveir
Building and Environment 46: 1899e1904. https://doi.org/10.1016/j.buildenv.2011.03.010 24.
In Chung, Byunghong Lee, Jiaqing
He, Robert PH Chang and Mercouri G
Kanatzidis. All-solid-state dye-sensitized solar cells with high efficiency
(2012) Nature 485: 486-489 https://doi.org/10.1038/nature11067 25.
Kroon JM. ENK6-CT2001-00575
NANOMAX 8-02-2005. 26.
S Karuppuchamy, K Nonomura, T
Yoshida, T Sugiura and H Minoura. Cathodic electrodeposition of oxide
semiconductor thin films and their application to dye-sensitized solar cells
(2001) Solid State Ionics 151: 19-27 https://doi.org/10.1016/S0167-2738(02)00599-4 27.
Anders Hagfeldt, Gerrit Boschloo,
Licheng Sun, Lars Kloo and Henrik Pettersson. Dye-Sensitized Solar Cells (2010)
Chem Rev 110: 6595-6663. DOI: 10.1021/cr900356p 28.
Michael Grätzel. Review
Dye-sensitized solar cells (2003) Elsevier J Photochemistry and Photobiology C:
Photochemistry Reviews 4: 145-153. doi:10.1016/S1389-5567(03)00026-1 29.
Md K Nazeeruddin, Etienne
Baranoff and Michael Gratzel. Dye-sensitized solar cells: A brief overview
(2011) Elsevier Solar Energy 85: 1172-1178. https://doi.org/10.1016/j.solener.2011.01.018 30.
Monzir S Abdel-Latif, Mahmoud B
Abuiriban, Taher M El-Agez, et al. Dye-Sensitized Solar Cells Using Dyes
Extracted From Flowers, Leaves, Parks, and Roots of Three Trees (2015) Int J
Renewable Energy Res. 31.
Kohjiro Hara and Hironori
Arakawa. Dye-sensitized Solar Cells. National Institute of Advanced Industrial
Science and Technology (AIST), Tsukuba, Japan. 32.
Yasuo Chiba, Ashraful Islam, Yuki
Watanabe, Ryoichi Komiya and Naoki Koide. Dye-Sensitized Solar Cells with
Conversion Efficiency of 11.1% (2006) Japanese J Appl Physics 45: L638–L640. https://doi.org/10.1143/JJAP.45.L638 33.
David Riehm. Improving
Dye-Sensitized Solar Cell Efficiency by Modification of Electrode Surface
Charge. 34.
Michael Gra1tzel. Solar Energy
Conversion by Dye-Sensitized Photovoltaic Cells (2005) Inorg Chem 44:
6841-6851. DOI: 10.1021/ic0508371 35.
Michael Grätzel.
Review Dye-sensitized solar cells (2003) Elsevier J Photochemistry and
Photobiology C: Photochemistry Reviews 4: 145-153.
doi:10.1016/S1389-5567(03)00026-1 36.
Michael Grätzel. Conversion of
sunlight to electric power by Nano crystalline dye-sensitized solar cells
(2004) Elsevier Journal of Photochemistry and Photobiology A: Chemistry 164:
3-14. https://doi.org/10.1016/j.jphotochem.2004.02.023 37.
Brian E Hardin, Henry J Snaith
and Michael D McGehee. The renaissance of dye-sensitized solar cells (2012)
Nature photonics 6: 162-169. https://doi.org/10.1038/nphoton.2012.22 38.
H Chang, TL Chen, KD Huang, SH
Chiena and KC Hung. Fabrication of highly efficient flexible dye-sensitized
solar cells (2010) Elsevier 504S: S435-S438. http://dx.doi.org/10.1016/j.jallcom.2010.02.044 39.
Tamotsu Horiuchi, Hidetoshi
Miura, Kouichi Sumioka and Satoshi
Uchida. High Efficiency of Dye-Sensitized Solar Cells Based on Metal-Free
Indoline Dyes (2004) J Ame Chemical
Society 126:12218-12219. https://doi.org/10.1021/ja0488277 40.
Tzi-Yi Wu, Ming-Hsiu Tsao, Fu-Lin
Chen, Shyh-Gang Su and Cheng-Wen Chang. Synthesis and Characterization of
Organic Dyes Containing Various Donors and Acceptors (2010) Int J Mol Sci 11:
329-53. https://doi.org/10.3390/ijms11010329 41.
Seigo Ito, Shaik M. Zakeeruddin,
Robin Humphry-Baker, Paul Liska, Raphaël Charvet, et al. High-Efficiency
Organic-Dye-Sensitized Solar Cells Controlled by Nanocrystalline-TiO2
Electrode Thickness (2005) Communications 18: 1202-1205 https://doi.org/10.1002/adma.200502540 42.
Kohjiro Hara, Tadatake Sato,
Ryuzi Katoh, Akihiro Furube, Yasuyo Ohga, et al. Molecular Design of Coumarin
Dyes for Efficient Dye-Sensitized Solar Cells (2003) J Phys Chem B 107:
597-606. DOI: 10.1021/jp026963x 43.
Qing Wang, Jacques E. Moser and
Michael Graltzel. Electrochemical Impedance Spectroscopic Analysis of
Dye-Sensitized Solar Cells (2005) J Phys Chem B 109: 14945-14953. http://dx.doi.org/10.1021/jp052768h 44.
Matt Law, Lori E. Greene, Justin
C. Johnson, Richard Saykally and Peidong Yang. Nanowire dye-sensitized solar
cells (2005) Nature Materials 4: 455-459 https://doi.org/10.1038/nmat1387 45.
Amaresh Mishra, Markus KR Fischer
and Peter B_Uerle. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From
Structure, Property Relationships to Design Rules (2008) Angew Chem Int Ed Engl
48: 2474-2499 https://doi.org/10.1002/anie.200804709 46.
Qifeng Zhang, Christopher S.
Dandeneau, Xiaoyuan Zhou, and Guozhong Cao. ZnO Nanostructures for
Dye-Sensitized Solar Cells (2008) Advanced materials 21: 4087-4108 https://doi.org/10.1002/adma.200803827 47.
Diah Susantia, Maula Nafi,
Hariyati Purwaningsiha, Rindang Fajarin and George Endri Kusuma. The
Preparation of Dye Sensitized Solar Cell (DSSC) from TiO2 and
Tamarillo Extract (2014) Elsevier Procedia Chemistry 9: 3-10. https://doi.org/10.1016/j.proche.2014.05.002 48.
Jun-HoYum, Peter Chen, Michael
Grtzel and Mohammad K Nazeeruddin. Recent Developments in Solid-State
Dye-Sensitized Solar Cells (2008) ChemSusChem 1: 699-707 https://doi.org/10.1002/cssc.200800084 49.
Tomohiro Nagata and Hirohiko
Murakami. Development of Dye-sensitized Solar Cells (2009). 50.
Kazuharu Suzuki, Makoto
Yamaguchi, Mikio Kumagai and Shozo
Yanagiday. Application of Carbon Nanotubes to Counter Electrodes of
Dye-sensitized Solar Cells (2003) Chemistry Letters 32: 1. http://dx.doi.org/10.1246/cl.2003.28 51.
Lukas Schmidt-Mende and Michael
Gratzel. TiO2 pore-filling and its effect on the efficiency of
solid-state dye-sensitized solar cells (2006) CH-1015 Lausanne, Switzerland. 52.
Fan-Tai Kong, Song-Yuan Dai and
Kong-Jia Wang. Review of Recent Progress in Dye-Sensitized Solar Cells (2007)
Advances in optoelectronics http://dx.doi.org/10.1155/2007/75384 53.
Zhong-Sheng Wang, Hiroshi
Kawauchi, Takeo Kashima and Hironori
Arakawa. Review significant influence of TiO2 photo electrode
morphology on the energy conversion efficiency of N719 dye-sensitized solar
cell (2004) Coordination Chemistry Reviews 248 1381-1389. https://doi.org/10.1016/j.ccr.2004.03.006 54.
Qamar Wali, Azhar Fakharuddin and
Rajan Jose. Tin oxide as a photo anode for dye-sensitized solar cells: Current
progress and future challenges (2015) J Power Sources 293: 1039e1052. https://doi.org/10.1016/j.jpowsour.2015.06.037 55.
M. Grätzel. Nature (2001). 56.
http://www.sony.co.jp/Products/SCHP/cx_pal/vol80/pdf/sideview80.pdf 57.
Norasikin A. Ludin, A.M.
Al-Alwani Mahmoud, Abu Bakar Mohamad, Abd. Amir H. Kadhum, Kamaruzzaman Sopian,
et al. Review on the development of natural dye photosensitizer for
dye-sensitized solar cells (2014) Renewable and Sustainable Energy Reviews 31:
386-396. https://doi.org/10.1016/j.rser.2013.12.001 58.
Jiawei Gong, Jing Liang and K
Sumathy. Review on dye-sensitized solar cells (DSSCs): Fundamental concepts and
novel materials (2012) Renewable and Sustainable Energy Reviews 16: 5848-5860. https://doi.org/10.1016/j.rser.2012.04.044 59.
Jason B Baxter. Commercialization
of dye sensitized solar cells: Present status and future research needs to
improve efficiency, stability, and manufacturing (2012) J Vacuum Sci Tech A30:
020801. https://doi.org/10.1116/1.3676433 60.
Nilofar Asim, Kamaruzzaman
Sopian, Shideh Ahmadi, Kasra Saeedfar, M.A. Alghoul, et al. A review on the
role of materials science in solar cells (2012) Elsevier 16: 5834–5847. https://doi.org/10.1016/j.rser.2012.06.004 61.
Adedokun Oluwaseun, Titilope
Kamil and Awodugba Ayodeji Oladiran. Review on Natural Dye-Sensitized Solar
Cells. Int J eng tech. 62.
Jeong-Hwa Kim, Dae-Hwan Kim,
Kang-Pil Kim, Dong-Hwan Jeon and Dae-Kue Hwang. Enhancement of the light
harvesting efficiency in a dye-sensitized solar cell by a patterned reflector
(2013) Thin Solid Films 546: 326-330. https://doi.org/10.1016/j.tsf.2013.03.062 63.
Hironori Arakawa. Recent Advances
in Research and Development for Dye-Sensitized Solar Cells II, CMC Publishing,
2007. 64.
Monishka Rita Narayan. Review:
Dye sensitized solar cells based on natural photosensitizers (2012) Renewable
and Sustainable Energy Reviews 16: 208-215. https://doi.org/10.1016/j.rser.2011.07.148 65.
Michal Sokolský and Július Cirák.
Dye-Sensitized solar cells: Materials and Processed (2010) Acta Electrotechnica
et Informatica10: 78-81. 66.
Umer Mehmood, Saleem-ur Rahman,
Khalil Harrabi, Ibnelwaleed A Hussein and Reddy BVS. Recent Advances in Dye
Sensitized Solar Cells (2014) Hindawi Publishing Corporation Advances in
Materials Science and Engineering 18: 155-162. https://doi.org/10.1016/j.mattod.2014.09.001 Andualem A, School of
Materials Science and Engineering, Jimma University, Jimma, Ethiopia,
E-mail: antenehanduale@ymail.com
Andualem
A, Demiss S. Review on Dye-Sensitized Solar Cells (DSSCs) (2018) J
Heterocyclics 1: 29-34 Review on Dye-Sensitized Solar Cells-(DSSCs)
Anteneh Andualem, Solomon Demiss
Abstract
Full-Text
Introduction
DSSCs
Materials
Dye Sensitizer
Metal Complex Sensitizers
Liquid Electrolytes
The general chemical reactions, which take part in all the processes, described
as follow [11,13,18]:
In general, working principles of DSSCs are distinct from other classes of
solar cells as the three key processes, i.e., light absorption and the
subsequent generation of electric charges, electron transport, and hole
transport are directed through three materials, thereby making them highly
interfacial devices [54].
The DSSC performance also depends on the film morphology Nanoparticles are
essential to increase surface area, and hence, amount of dye, while large
particles are required to enhance absorption of red light through light
scattering. It is impossible to increase surface area and light scattering
simultaneously, because they oppose each other. Therefore, there must be a
balance between them. Such a balance was well controlled by tuning the layer
structure, and an energy conversion efficiency of 10.2% was obtained using a
multilayer structure. The multilayer structure is also suitable for other dyes
in terms of improving light harvesting efficiency, and hence, photocurrent. In
order to scatter the red light more efficiently, a more sophisticated
multilayer structure with gradually increased particle size from the most-inner
layer is desirable [53].
The performance of a DSSC is explained by its I-V characteristics which is
attained from parameters such as short-circuit current Isc and open
circuit voltage Voc and Fill factor (FF) . The ff can be given by
the equation:
Advantages of
DSSCs
Capable of production in a simple way: Dye-sensitized
solar cells require no vacuum system for manufacturing, and thus have an
essential advantage in terms of production cost [63]. It reduces manufacturing
cost by 1/5 to 1/10 as compared to silicon solar cells production cost [22, 23
and 55].
Lighter weight:
Plastic
substrates can be used to minimize the weight of solar cells and panels. Dou to
its light weight, dye-sensitized solar cells can be installed in locations
where appearance is important and other solar cells are not applicable, such as
the glass panes and outer and inner walls of a building, the sunroof and outer
panels of an automobile, and
the enclosure of a hand phone. This allows the creation of new markets with
high demand [55,56.58] (Figure 4).Conclusion

*Corresponding
author:
Citation: