Introduction
Four novel functionalized microparticles of graphite have been prepared directly from graphite by the modified approach of mechanoactivated reactive exfoliation or by refunctionalization. Carbon fibers are known to increase strength of polymer composite materials, especially for the applications where high rigidity and low weight are of value. The interface between carbon fibers and the polymer matrix that surrounds those plays an important role in the material properties of the composite material [1,2]. the low affinity of most plastic matrices to the crystalline graphitic domains of carbon fibers is usually remediated by coating the fibers with sizing-a polymer with the functional groups improving adhesion.
A better control over the surface of carbon and greater versatility of its properties can be achieved by the direct introduction of functional groups. While covalent modification of low reactive graphitic basal planes is difficult, their ability for π-π stacking can be utilized for direct functionalization. Thus, the pyrene moiety was used as an anchor for introduction of N-hydroxysuccinimide or gold nanoparticles to carbon nanotubes [3,4]. The availability of the graphitic basal planes in the edge-functionalized particles of graphene makes them a promising material for rapid and versatile functionalization of carbon fibers tailored to the polymer matrix in the desired composite. Considering the known improvement of the mechanical performance of carbon fiber/epoxy composites by grafting graphene oxide into the fibers, development of versatile functionalized graphenes presents a significant practical interest [5].
Besides of a rather expensive synthesis of graphene by the chemical vapor deposition, a variety of top-down methods exist as well. Graphite oxidation and subsequent reduction produce a material called reduced graphene oxide, which is a graphene with more defects [6,7].
In 2013, Baek and coauthors have reported a method that exfoliates graphite into graphene and adds functionalization at the same time due to the Diels-Alder addition of maleic anhydride or maleimide to the diene moieties of the mechanoactivated graphitic layers [7]. While Seo JM, et al., refers to the particles as graphene, their structure seems to better fit the category of micro particles of graphite [7]. This solvent-free mechanochemical procedure produces multilayered graphite microparticles functionalized at the edge of their graphitic planes at the gram scale, and requires inexpensive reagents and a ball mill. However, only two functional groups (anhydride and imido-group) have been introduced to the micro particles.
Here we report facile preparation of three new types of edge-functionalized graphite micro particles and their adhesion to unsized and sized industrial carbon fibers along with a series of existing carbonaceous materials. The hexadecylimido- and 1,6-hexane-bis-imido-functionalized micro particles of graphite were prepared by the expansion of the mechanoactivated chemical exfoliation of graphite to the N-hexadecylmaleimide and 1,6-dimaleimidehexane dienophiles. The (2Z)-4-(6-aminohexyl-1-amino)-4-oxobut-2-enoic acid functional group was introduced to the anhydride-functionalized micro particles of graphite by their reaction with 1, 6-diaminohexane.
Experimental Part
Materials and equipment
1-Hexadecylamine, triethylamine and diisopropyl carbodiimide (DIC)from Alfa Aesar, graphite powder with a diameter of<20 µm - from Sigma-Aldrich, Fmoc-6-aminohexanoic acid (Fmoc-Ahx-OH) - from Chem-Impex International, maleic anhydride, acetic anhydride, nickel (ll) acetate, acetic acid, hexane, methanol, N-hydroxybenzotriazole (HOBt), maleimide, N,N-dimethylformamide (DMF), 1,6-diaminohexane and dichloromethane (DCM) -from Oakwood Chemicals. Graphene: Unmodified GraphenX™ graphene was purchased from www.cheaptubes.com and used as-is without further modification. The carbon fibers of the SGL APS type were provided by SGL Corporation. XRD analysis was carried out using a monochro XRD instrument with energy of 40.0 kV by Rigaku Ultima IV. SEM measurements were carried out using InLense and SE2 detector with an energy of 1.50 to 3.00 kV by SIGMA ZEISS. FTIR measurements were carried out from 400 to 4000 cm-1 by Perkin Elmer. The ball millings for graphite functionalization were carried out by Planetary Ball Mill (PBM-04, Micro Nano Tools). XPS measurements were carried out using monochromatic Al Ka x-ray with energy of 1486.6 eV by Thermo Scientific K-alpha+ XPS. The hydrodynamic particle sizes have been analyzed by the DLS (Dynamic Light Scattering) method with Zetasizer (model: Malvern Zetasizer Nano Series). Ultrasonication was performed in a Fisher Scientific FS20H ultrasonic bath. UV-vis absorption spectra were measured on Varian Cary 50 UV-Vis spectrophotometer. Braunauer-Emmett-Teller (BET) analysis for the determination of surface area was done by Quantachrome Novawin (version 11.03). 1H NMR spectra were recorded on a Brucker 400 MHz spectrometer in CDCl3 and TMS (tetramethylsylane) as an internal standard.
Synthesis of dienophiles
N-hexadecylmaleimide was prepared by a modified known procedure [8]. Briefly, 1-Hexadecylamine (22.99 g, 0.095 mol) and maleic anhydride (10.51 g, 0.107 mol) were refluxed in 150 mL glacial acetic acid until completion (approximately 6 h, monitored by 1H NMR). The reaction mixture was allowed to cool to room temperature and poured into 2000 mL of cold deionized water with stirring. This mixture was stirred for 6 h and filtered through filter paper. The solid material is dried in air, broken into a powder, washed repeatedly with deionized water to remove residual acetic acid, and dried in a vacuum desiccator. Yield 28.9 g (84.0%). M.p. 45-55°C; lit. M.p. 103-105°C. 1H NMR (CDCl3, 400 MHz): δ 6.67 (s, 2H), δ 3.50 (t, 2H, J= 7.2 Hz), δ 1.63 (m, 28H, 7.56), δ 0.87 (t, 3H). By NMR, the purity of the compound is estimated as ~96%. The product was used without further purification [8].
1,6-Bismaleimidohexane was prepared by a modified known procedure. briefly, maleic anhydride (49.0 g, 0.5 mol) was dissolved in 150 mL DMF in a 500 mL round bottom flask at magnetic stirring. Next, 1,6-diaminohexane (29.05 g, 0.25 mol) was added to the flask at stirring. The solution slightly heated up and turned translucent yellow [9].
The temperature was maintained at 90°C for 30 min on a hot plate. Then acetic anhydride (94 mL, 0.99 mol), triethylamine (13.8 mL, 0.099 mol), and nickel (II) acetate tetra hydrate (0.5 g, 0.0028 mol) were added within 5 min. After the temperature was maintained for 30 min, the reaction mixture was allowed to cool to 40° C and was poured to 2L of deionized water in an ice bath. The brown precipitate was filtered, washed with 1L of water in small portions, dried at 80°C in air for 3 days. The solid mass was crushed into a powder, stirred with 2L of water, filtered, and dried at 80°C in air for 4 h. Yield 120.9g (88%). MP. 125-130°C, lit. m.p. 144°C. 1H NMR (CDCl3, 400 MHz): δ 6.70 (s, 4H), δ 3.52 (t, 4H, J=7.4 Hz), δ 1.59 (m, 4H), δ 1.32 (m, 4H). By NMR, the purity of the compound is estimated as ~95 % [9].
Preparation of graphite micro particles
In a typical procedure, 3 g of graphite and 6 g of a dienophile (maleic anhydride, maleimide, N-hexadecylmaleimide or 1,6-dimaleimidohexane) were ground in a 50 mL stainless steel jar using a planetary ball mill operating at 500 rpm for 48 h [7]. The reaction mixture was washed with a solvent (hexane for N-hexadecylmaleimide, methanol for maleimide, methanol for Bis-maleimide, methanol for maleic anhydride) on a fritted glass funnel. The graphite micro particles were dried, weighed, and washed again. The washing cycles continued until the constant weight of the product (typically, 3-6 mL of each solvent was used in each of 3 washing cycles). The extent of functionalization was estimated by the gravimetrically measured weight gain [7].
Non-functionalized graphite micro particles: Graphite was processed in the same ball mill under the same conditions but without a dienophiles to produce the control material GC.
Pre-treatment and non-covalent modification of carbon fibers: Unsized carbon fibers were prepared by heating chopped 1-2 bundles of sized fibers (APS) in a nitrogen purged furnace at 500°C for 5 h to remove any surface coating. The fibers to be modified (100 mg) were sonicated in a suspension of 100 mg of graphite micro particles in 10 mL of a solvent for 3 min, filtered and washed with 5 mL of the same solvent.
Preparation of graphene quantum dots
These particles were made using a modified method based on Ye, R et al. Briefly, a 300 mg portion of anthracite coal was ultrasonicated in 60 mL of 96% H2SO4 and 20 mL 60% HNO3 for 6 h, then heated to 100°C for 24 h with continuous stirring. After cooling to room temperature, the solution was poured over 100 g of ice. The pH of the solution was brought to ~7 by a 3M (and at the end – 0.01M) sodium hydroxide solution. The solution was decanted, passed through a 0.45 µm PTFE syringe filter, dialyzed through a 3500 Da membrane against deionized water for 7 days. The solvent was removed in vacuum to produce the material GQD [10].
Preparation of graphite micro particles functionalized with (2Z)-4-(6-aminohexyl-1-amino)-4-oxobut-2-enoic acid (GAM)
A mixture of 3 g of maleic anhydride functionalized graphene (GMA) and 33 g of 1,6-diaminohexane was stirred in 100 ml of DMF at 110oC for 20 min, next at 90oC for 2h. The mixture was cooled to room temperature and centrifuged. The precipitate was washed with DMF (3X13 mL) and dried at room temperature in air, yield 84% (GAM).
Determination
of amino-groups on carbon
It was performed by the modified procedure of determination of amino-groups on carbon
nanofibers. Specifically; 150 mg of the aminated
graphite (GAM), 175 mg (0.5 m mol) of 6-(Fmoc-amino) hexanoic acid (Fmoc-Ahx-OH),
and 67.5 mg (0.5 m mol) of N-hydroxybenzotriazole (HOBt) were mixed in a 15 mL
vial. Then a solution of 80 µL (0.5 m mol) of diisopropylcarbodiimide (DIC) in
1 mL of DMF was added. The mixture was magnetically for 12 h and filtered. The
solid material on the filter was washed with DMF (3 mL), methanol (3 mL), DCM
(3 mL), and dried in air overnight. A 20 mg sample of the dried material was shaken
for 45 min with a 20:80 (v/v) mixture of piperidine and DMF and centrifuged at
10,000 rpm for 4 min. The centrifugate was diluted X20 (dilution factor, DF)
with 20:80 (v/v) mixture of piperidine and DMF, and the absorbance was measured
at 290 nm [11].
The density of amino-groups was calculated using the following equation [11]:
|
Density of NH2 (mmol/g) = |
Absorbance |
*Dilution Factor (DF) |
|
Mass of the material * 1.75 |
DLS measurements: A 5 mg sample of a material was suspended in 10 mL of deionized water by ultra-sonication for 10 min, decanted, and measured on the Zetasizer instrument.
XRD analysis
A 4-6 mg of a sample was placed on the sample holder and measured from 5 to 80 degree with a scan speed of 0.300 deg/min and sample width of 0.0200 degree. The data was processed by PDXL2 software.
SEM imaging
A few drops of a suspension of ~5 mg of a sample in 10 mL of hexane was affixed on a silicon wafer with a carbon double sticky tape and dried on air. The sample was rinsed with hexane and dried on air again.
FTIR analysis
A 3-5 mg of a sample was ground in a mortar with ~50 mg of dry KBr and pressed into transparent KBr pellets. The transmission data in the 400-4000 cm-1 range was processed by Spectrum2 software.
XPS analysis
A 2-5 mg sample was attached to a conductive stage by double-sided carbon tape and held at 5*10-8 mbar for 4 h before the experiment. The data were processed by the Advantage software package.
Surface analysis
An approximately 0.05 g sample of a material was evacuated and pre-heated at 100oC for 5.5 to 14 h. Next, the sample was cooled by liquid N2, and the adsorption of N2 gas was measured at an array of pressures. The surface area was calculated by the Brunauer-Emmett-Teller (BET) analysis, and the Barrett, Joyner and Halenda (BJH) method was used to calculate the average pore size and volume.
Results and Discussion
Functionalized graphite microparticles
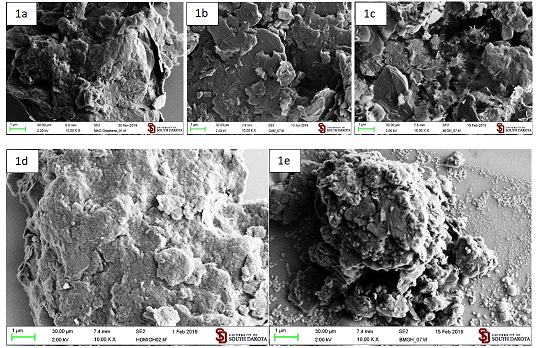
Preparation of functionalized graphite microparticles by ball milling with a dienophile: The graphite microparticles functionalized with maleimide (GMI, Figure 1b) were prepared in air with maleimide as the dienophile, which produced 4.5% weight gain. The extent of functionalization depended on the reaction conditions. When the ball milling was performed in air, the weight gain was 4.5%, increased when the reaction was run under nitrogen (16% weight gain) and reached 27% under 74 millitorr vacuum. The SEM analysis did not reveal any substantial influence of the reaction atmosphere on the size and morphology of graphite microparticles. All other functionalized microparticles of graphite have been prepared under vacuum to minimize potential oxidation by the atmospheric and adsorbed air.
The graphite microparticles functionalized with maleic anhydride (GMA, Figure 1a) were prepared under 80 millitorr vaccum with maleic anhydride as the dienophile, which produced 7.2% weight gain.
The graphite microparticles functionalized with N-hexadecylmaleimide (GHDMI, Figure 1c) were prepared under 84 millitorr vacuum conditions with N-hexadecylmaleimide as the dienophile, which produced 5.4% weight gain.
The graphite microparticles functionalized with 1,6 dimaleimidohexane (Gbis, Figure 1d) were prepared under 80 millitorr vacuum (weight gain 6.2%) or under nitrogen (weight gain 13%) with 1,6-dimaleimidohexane as the dienophile. Thus, we optimized the conditions of the mechanochemical synthesis and expanded its applicability to N-substituted maleimide.
Graphite microparticles functionalized with (2Z)-4-(6-aminohexyl-1-amino)-4-oxobut-2-enoic acid
(GAM)
This aminated material was prepared by re-functionalization of the maleic anhydride derivative GMA with excess of 1,6-diaminohexane. SEM images of functionalized graphite microparticles prepared by Mechanoactivation (a-d) are presented in Figure 1.
The direct quantitative analysis of amino-groups on carbons is difficult because carbon absorbs and catalyzes degradation of most of the dyes involved in the analysis. Therefore, we employed the Fmoc-based analytical protocol [11] (Figure 2).
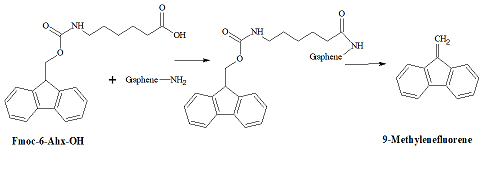
Figure 2: Analysis for the surface amino groups.
First, the amino-groups on the carbons surface are covalently coupled with a chromophore-containing Fmoc-6-Ahx-OH. Next, after removal the excess of Fmoc-6-Ahx-OH, cleavage of the Fmoc-moiety with piperidine releases into solution one molecule of 9-methylenefluorene per each surface amino-group, which is determined photo metrically. In the aminated graphite microparticles GAM, the density of amino-groups determined by the Fmoc method was 0.22 m mol/g [11].
FT-IR Spectroscopy
The surface functional groups have been identified by FT-IR spectroscopy. The strong adsorption at ~1700 cm-1 is characteristic to the stretching C=O vibration in the material functionalized with imido-groups (GMI), the material functionalized with maleic anhydride (GMA), and its derivative open with a diamine (GAM) (Figure 3). For the maleic anhydride derivative (GMA), the C=O vibration occurs below the characteristic for the anhydride 1800 cm-1 wavenumber. These points to the hydrolysis of the anhydride ring, which is consistent with the literature [7]. The presence of the absorption band at ~3098 cm-1, which is characteristic for C(sp2)-H stretching vibrations indicates that some molecules of maleic anhydride acylated OH- groups available at the oxidized edges of graphene layers. The absence of an unreacted phase of maleic anhydride was confirmed by the powder XRD analysis. No multiple reflections characteristic for maleic anhydride were observed [12]. The materials functionalized with weak acylating agents - maleimide derivatives did not show the C (sp2)-H band. A series of bands at ~1200-1285 cm-1 characteristic for the presence C-O bonds is especially strong for the maleic anhydride derivative. The nitrogen-containing GMI shows a weak shoulder at ~1345 cm-1 due to C-N absorption, which is barely visible in GAM. The strong ~1580-1600 cm-1 bands of the stretching vibrations of non-symmetrical C=C in GMA are much weaker in the nitrogen-containing materials GMI and GAM and almost not visible in the materials GHDMI and Gbis (Figures 3 and 4).
Introduction of sp3-carbons to the materials in the processes of cycloaddition and a diamine acylation leads to the C-H stretching vibrations at ~2850-2968 cm-1. Broad absorptions at ~3000-3500 cm-1 characteristic for -OH and -NH stretching vibrations are especially strong for the aminated material (GAM) (Figure 3).
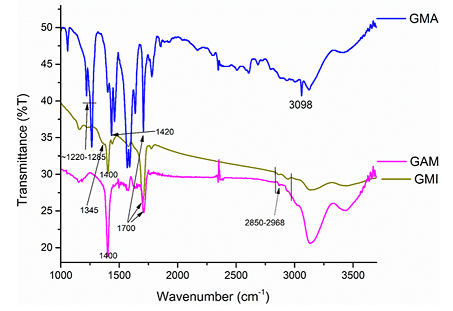
Figure 3: FTIR-spectra of known (GMA, GMI) functionalized graphite microparticles and the aminated material (GAM).
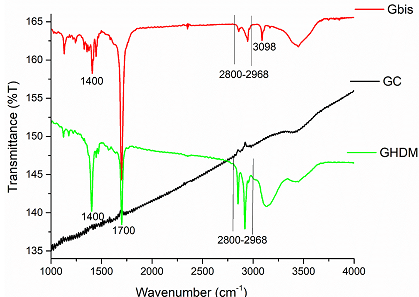
Figure 4: FTIR-spectra of new functionalized graphite microparticles prepared directly by Mechanoactivation (GHDMI, Gbis). The ball milled non-functionalized graphite (GC) is a reference.
The absorption patterns of new functionalized graphite microparticles directly synthesized by ball milling of graphite with long-chain derivatives of maleimide are similar to the materials GMA, GMI and GAM. Due to the presence of long alkyl chains, the new materials show prominent absorptions at ~2850-2968 cm-1, which is especially strong for the GHDMI derivative, containing hexadecyl groups (Figure 4). The material Gbis functionalized with a molecule with two maleimide moieties shows an absorption band at ~3098 cm-1, which is characteristic for C (sp2)-H stretching vibrations. These points to the presence of free maleimide groups in the graphite microparticles, which may enable their subsequent cross-linking and re-functionalization.
Powder XRD
According to the XRD diffraction patterns of the functionalized graphite microparticles, the spacing between graphene layers (~3.5Å, 2θ=26.4o) matches the one in the parent graphite, which is consistent with their multilayer morphology. This allowed us to categorize the particles as multilayered particles of graphite. This lack of other reflections confirms that the material does not contain any maleic anhydride impurity, which is known to produce a multitude of reflections scattered all over the spectral range [12,13] (Figure 5).
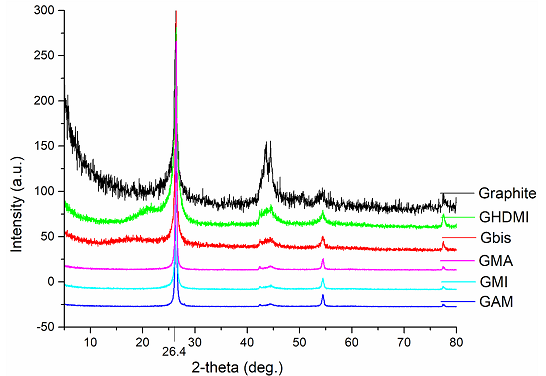
Figure 5: XRD patterns of functionalized multilayered graphene microparticles.
Surface analysis
The microparticles of Gbis (more projections are given in Figure 6a and Figure 6b preserve the high porosity of the material and their morphology is very similar to that of the control material GC (ball milling without a dienophile) (Figure 6).

Figure 6: SEM micrographs of a, b Gbis microparticles. c. Control material GC.
This is in contrast with all other low porous functionalized materials (Figure 1). The retention of the morphology in Gbis could be attributed to the cross-linking ability of 1,6-dimaleimidohexane. The surface analysis results of the new materials are presented in Table 1.
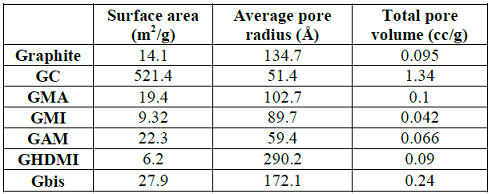
Table 1: Surface analysis of functionalized graphite microparticles.
The kinetic energy generated in the mill breaks graphite chunks into small particles, which drastically increases the surface area and the total pore volume. However, the resulting GC material is not functionalized, and its pores are significantly shrunk comparing with graphite. The increase of the surface area of graphite upon mechanoactivated functionalization is much less pronounced while functionalization with HDMI (N-Hexadecylmaleimide) even decreases this parameter. Among all functionalized microparticles of graphite, the Gbis material has the highest surface area and total pore volume, perhaps, due to the cross-linking by the bifunctional Bis-maleimide. The drastic increase of solvation of Gbis by water and methanol compared with even more polar GMA and GAM further points at the unique properties of the cross-linked Gbis, which contains both hydrophobic alkyl chains and reactive maleimide-groups while maintaining the pore size of the bulk graphite.
Dispersibility in solvents
The type of the functional group on the graphite microparticles significantly affected their dispersibility in DCM, methanol, and water, which was estimated by sonicating 10 mg of the microparticles in 5 mL of the solvent for 6 min and observing the suspension for 1 week.
The control material GC (ball mill grounded graphite microparticles) was suspended to test their stability in different solvent systems. The suspended GC was mostly stable in water up to 3 days while in hexane it completely precipitated after 40 minutes, and in DCM it took 60 minutes for complete precipitation.
The microparticles MA and MI prepared by the known method did not form a stable suspension with water, hexane, or DCM. However, relatively stable suspensions (not completely separated after 1 day) were obtained in methanol. The suspension of control GC in methanol was stable up to 3 days [1].
The most hydrophobic GHDMI containing long alkyl chains (16 carbons), expectedly failed to form a suspension in water. It is suspension in methanol was stable for 1 day and completely separated in 1 week. GHDMI was best solvated by DCM, which produced a suspension still stable after 1 week. The least polar hexane, however, did not sustain a stable suspension with neither GHDMI nor any other functionalized microparticles. Therefore, introduction of a long alkyl chain to a maleimide-functionalized microparticle of graphite significantly increased its dispersibility in methanol, and especially – in DCM.
In the material Gbis, the maleimide moieties are cross-linked with a short alkyl chain (6 carbons). The suspension of Gbis in DCM was less stable than that of GHDMI: while persisting for 1 day, it completely separated in 1 week. However, dispersibility of Gbis in methanol noticeably improved compared with GHDMI: the Gbis suspension in methanol still persisted after 1 week. Remarkably, Gbis was the only type of functionalized microparticles that was able to form an aqueous suspension still stable after 1 day. Although the suspension completely separated in 1 week, its stability was sufficient for the industrial modification of carbon fibers using a green non-toxic and non-flammable dispersant. The drastic increase of solvation of Gbis by water and methanol compared with even more polar GMA and GMI is consistent with the higher porosity of Gbis and the cross-linking ability of 1,6-dimaleimideohexane.
Due to the presence of acidic groups on the graphitic surface, the stability of the aqueous suspensions was higher at higher pH values, adjusted by aqueous HCl and NaOH. While after 7 h, the aqueous suspension of Gbis at pH = 3 completely separated, the particles mostly remained suspended at pH = 7 to 11.
The observed dispersibility of the materials was consistent with the pH-dependent particle sizes measured by DLS. Thus, changing pH from 7.0 to 5.0 led to the substantial increase of the average particle size from 367 to 526 nm. This makes the Gbis material a candidate for a pH-controlled drug delivery scaffold (Table 2).

Table 2: pH-Effect on the size of Gbis particles determined by DLS.
Modification of carbon fibers with known carbon particles
For comparison, we examined adhesion of four types of carbon particles (graphite, commercial GraphenX, carbon quantum dots GQD,and graphite milled without a dienophile (GC)) to carbon fibers [10] (Figures 6c and 7).

Figure 7: Carbon particles: (a) unmodified graphite, (b) Graphene™, (c) Graphene quantum dots GQD.
The treatment of carbon fibers with an aqueous suspension of carbon quantum dots (GQD) led to a flat positioning of the dots over the surface of fibers (Figure 8d), while the commercial graphene (GraphenX) formed clusters just loosely associated with the carbon fibers surface (Figure 8b). Commercial graphite did not coat carbon fibers from its aqueous suspension at all (Figure 8c). However, it did deposit loosely associated clusters from its suspension in hexane (Figure 8).
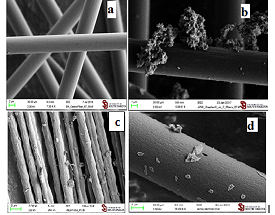
Figure 8: a. untreated carbon fibers. Carbon fibers treated with suspensions of: b. GraphenX in water; c. Graphite in hexane; d. Carbon quantum dots GQD in water.
The deposition of quantum dots on carbon fibers was additionally confirmed by their reduced concentration after exposure to the fibers. An aqueous suspension of carbon quantum dots was divided into two parts. After one part (30 mL) was exposed to carbon fibers (100 mg), concentration of carbon quantum dots was measured in both parts by UV-vis spectroscopy (Figure 9).
Concentration of GQD in the stock solution measured by complete evaporation of the solvent was 1.50 g/L. The concentration of GQD in the solution after exposure to carbon fibers was estimated by the Beer-Lambert Law at 260 nm as 1.39 g/L. Therefore, the amount of GQD deposited on 0.1 g of carbon fibers was (1.50 – 1.39) g/L X 0.030 L = 0.0033 g, which 3.3 wt%.
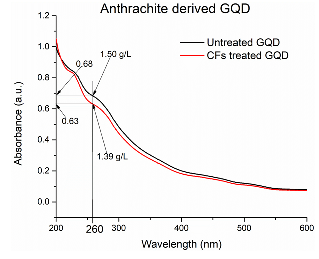
Figure 9: UV-vis-absorption by aqueous suspension of carbon quantum dots. Blue line–before exposure to carbon fibers. Red line– after exposure to carbon fibers.
Modification of carbon fibers with functionalized graphite microparticles
Modification of carbon fibers with graphite microparticles functionalized with maleic anhydride
Maleic anhydride functionalized graphite microparticles (GMA) coat carbon fibers by a simple dipping to their suspension in hexane (Figure 10).

Figure 10: Carbon fibers coated with graphite nanoparticles functionalized with maleic anhydride (GMA) in hexane.
Modification of carbon fibers with graphite microparticles functionalized with maleimide
The coating obtained from the maleimide functionalized graphite microparticles (GMI) seems to be denser and with fewer particles sitting on their edges (Figure 11).
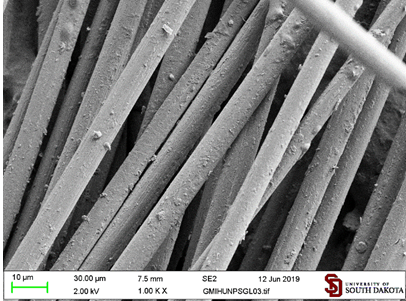
Modification of carbon fibers with graphite microparticles functionalized with hexadecylmaleimide
The coating by hexadecylmaleimide functionalized graphite microparticles (GHDMI) consists of long flakes almost completely covering the surface of fibers (Figure 12).
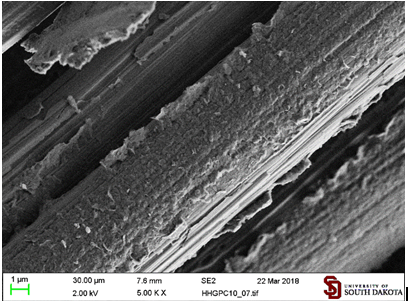
Figure 12: Carbon fibers coated by graphite microparticles functionalized with hexadecylmaleimide (GHDMI) from their hexane suspension.
Introduction of the GHDMI material to the surface of carbon fibers significantly affects its XPS spectrum. The surface elemental analysis of commercial carbon fibers reveals the presence of a noticeable amount of oxygen (C-86.08%, N-3.84%, O-10.08%) due to the plasma treatment routinely used to improve the adherence of fibers to the sizing (coating of carbon fibers before placing them to a composite material). The GHDMI – coated surface contains less oxygen due to the blockage by the long alkyl chains and more carbon because of the high carbon content of alkanes (C-89.98%, N-3.12%, O-6.91%).
Modification of carbon fibers with graphite microparticles functionalized with 1,6-dimaleimidohexane.
The treatment of carbon fibers with a hexane suspension of graphite functionalized with 1,6-dimaleimidohexane (Gbis) produced a dense coating of the fibers with the material (Figure 13).

Figure 13: Carbon fibers coated by graphite microparticles functionalized with 1,6-dimaleimidohexane (Gbis) from their hexane suspension.
The strongest effect of Gbis on the elemental composition of the surface is substantial increase of the nitrogen content due to the presence of two atoms of nitrogen in the 1,6-dimaleimidehexane linker (C-85.81%, N-4.92%, O-9.27%). The chemical environment around the atoms of carbon and oxygen on the surface of modified carbon fibers was examined by the deconvolution analysis of the 1s bands with the sp2 component fixed at 284.0 eV (Table 3).
The long alkyls chains in GHDMI introduce to the surface more C-C bonds while the highly oxidized C=O groups are blocked by the adsorption of the hydrophobic graphite particles, which is evident from the chemical environment changes around both carbons and oxygens.
This shifts the balance of the oxygen chemical environment from C=O to C-O groups. Similarly to GHDMI, the Gbis particles also introduce the C-C bonds to the surface. However, the graphene surface of the particles is not blocked by hexadecyl chain and introduces its own C=O groups to the surface.
Thus, despite the preferential adsorption of the particles on the C=O groups of the surface of carbon fibers, their contribution to the chemical environment both around carbons and nitrogens does not substantially decrease. The abundance of maleimido- in surface C=O groups in Gbis shows in the increased contribution of the sp2 component.
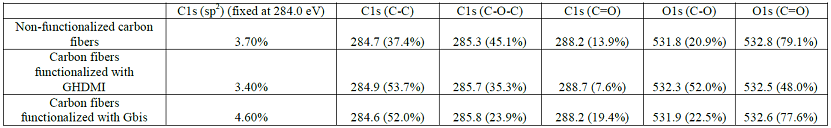
Table 3: Structural XPS analysis of modified carbon fibers.
Modification of carbon fibers with graphite microparticles functionalized with (2Z)-4-(6-aminohexyl-1-amino)-4-oxobut-2-enoic acid
We found that the aminated graphite microparticles efficiently adhere to carbon fibers from their DCM, hexane, or aqueous suspensions. The SEM images on Figure 14 show that both flat and edge areas of the particles demonstrate substantial affinity to the fibers.

Figure 14: Carbon fibers coated by the aminated graphite microparticles (GAM) suspended in (a) DCM; (b) Hexane; (c) Water.
Modification of sized carbon fibers with functionalized graphite microparticles
Modification of sized carbon fibers with graphite nanoparticles functionalized with hexadecylmaleimide: We found that the adhesive ability of graphite microparticles is not limited to carbon-carbon p-stacking interaction. The hydrophobic interaction seems to play a significant role as well. Thus, graphite microparticlres functionalized with N-hexadecylmaleimide (GHDMI) adheres to sized (coated with an organic polymer) carbon fibers upon dipping to their hexane suspension. The subsequent sonication for 10 min knocked off most of loosely attached particles, while the well-adhered particles were retained (Figure 15). A similar trend was observed for the suspension of GHDMI in DCM (Fig. 16).

Figure 15: Sized carbon fibers coated by the graphite microparticles functionalized with N-hexadecylmaleimide (GHDMI) suspended in hexane. (a) After regular sonication (3 min); (b) After additional 10 min of sonication.

Modification of sized carbon fibers with graphite microparticles functionalized with 1,6-dimaleimidohexane: Graphite microparticles functionalized with 1,6-dimaleimidohexane (Gbis) adheres to sized carbon fibers from its hexane and DCM suspensions. This produces a denser, more evenly distributed and more resistant to sonication coating than GHDMI (Figures 17 and 18).

Figure 17: Sized carbon fibers coated by the graphite microparticles functionalized with N-hexadecylmaleimide (Gbis) suspended in hexane. (a) After regular sonication (3 min); (b) After additional 10 min of sonication.
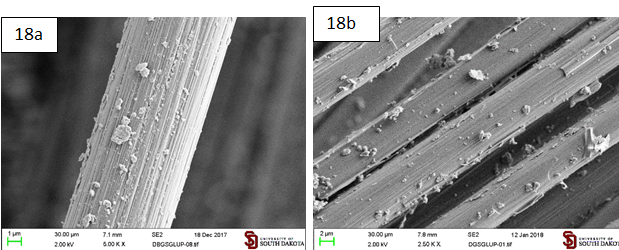
Figure 18: Sized carbon fibers coated by the graphite microparticles functionalized with N-hexadecylmaleimide (Gbis) suspended in DCM. a. After regular sonication (3 min); b. After additional 10 min of sonication.
The high Dispersibility of Gbis particles in water allowed for modification of carbon fibers directly by their aqueous suspension. This produces a less dense coating than suspensions in hexane and DCM, which is however resistant to sonication (Figure 19).

Figure 19: Sized carbon fibers coated by the graphite microparticles functionalized with N-hexadecylmaleimide (Gbis) suspended in water. a. After regular sonication (3 min); b. After additional 10 min of sonication.
Modification of sized carbon fibers with aminated graphite microparticles: The aminated graphite microparticles also retained their affinity to sized carbon fibers. The particles are loosely associating with the fibers mostly by the edges of particles (Figure 20).

Figure 20: Sized carbon fibers coated by the aminated graphite microparticles (GAM) suspended in (a) DCM; (b) Hexane; (c) Water.
The unmodified graphite particles (GC) obtained by simple ball milling of graphite can evenly coat both carbon fibers and sized carbon fibers by their aqueous suspension as well as the modified Gbis material. That coating, however, is lacking the functional groups introduced by functionalized graphite microparticles (Figure 21).
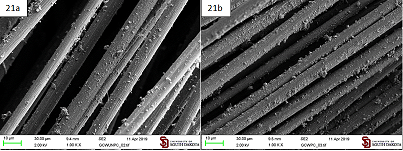
Figure 21: Coating produced by an aqueous suspension of unmodified graphite microparticles (GC) on a. Carbon fibers; b. Sized carbon fibers.
Thus, the affinity of functionalized graphite microparticles to carbon fibers is comparable with the affinity of less available graphene. Further, functionalization of the particles allows for the fine tuning of their affinity and other properties, which make them a viable alternative of graphene for many applications, such as composite films, wound healing, hair dyes, catalysts for green synthesis, and antioxidants [14-18].
Conclusions
Functionalized multi-layer graphite microparticles are able to coat both sized and unsized carbon fibers by simple dipping to their suspensions in various solvents including water. The produced coatings are resistant to ultrasonication. The ability to coat carbon fibers depends on both the type of functionalized particles and on the suspension solvent. Dispersibility of the Gbis material (which contains both hydrophobic alkyl groups and reactive maleimido-groups) in water and the particle sizes substantially changes between the pH values 7 and 5, which is promising in terms of the drug delivery applications. The results of this work will help efficiently incorporate carbon materials into the composite materials with a diverse range of applications.
The raw and processed data required to reproduce these findings are available to download from
https://data.mendeley.com/datasets/y5hkynnw6v/1
Acknowledgements
We thank the South Dakota Center for Composite and Nanocomposite Advanced Manufacturing (CNAM), the South Dakota Surface Engineering Research Center (SERC), the Department of Chemistry of The University of South Dakota, NSF-MRI grants CHE-1337707 (SEM/EDS instruments) and CHE- 1229035 (400 MHz NMR spectrometer). NASA EPSCoR grant NNX15AK54A for the financial support of this work.
The research was performed in part in the Nebraska Nanoscale Facility: National Nanotechnology Coordinated Infrastructure and the Nebraska Center for Materials and Nanoscience, which are supported by the National Science Foundation under Award ECCS: 1542182, and the Nebraska Research Initiative. We also thank Prof. Jong-Beom Baeks group for providing samples of two functionalized graphenes.
References
1. Ma Q, Gu Y, Li M, Wang S and Zhang Z. Effects of surface treating methods of high-strength carbon fibers on interfacial properties of epoxy resin matrix composite (2016) Applied Surface Sci 379: 199-205. https://doi.org/10.1016/j.apsusc.2016.04.075
2. Chen JR, Zhang Y, Wang D and Dai H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization (2001) J Am Chem Soc 123: 3838-3839. https://doi.org/10.1021/ja010172b
3. Salice P, Gambarin A, Daldosso N, Mancin F and Menna E. Noncovalent interaction between single-walled carbon nanotubes and pyrene-functionalized gold nanoparticles in water-soluble nanohybrids (2014) The J Phys Chem C 118: 27028-27038. https://doi.org/10.1021/jp505005e
4. Zhang X, Fan X, Yan C, Li H, Zhu Y et al. Interfacial microstructure and properties of carbon fiber composites modified with graphene oxide (2012) ACS Appl Mater Interfaces 4: 1543-1552.
https://doi.org/10.1021/am201757v
5. Ismach A, Druzgalski C, Penwell S, Schwartzberg A, Zheng M et al. Direct chemical vapor deposition of graphene on dielectric surfaces (2010) Nano Lett 10: 1542-1548. https://doi.org/10.1021/nl9037714
6. Zaaba NI, Foo KL, Hashim U, Tan SJ, Liu WW et al. Synthesis of graphene oxide using modified hummers method: solvent influence (2017) Procedia Engineering 184: 469-477. https://doi.org/10.1016/j.proeng.2017.04.118
7. Seo JM, Jeon IY and Baek JB. Mechanochemically driven solid-state diels-alder reaction of graphite into graphene nanoplatelets (2013) Chemical Sci 4: 4273. https://doi.org/10.1039/c3sc51546j
8. Han J, Huang X, Sun L, Li Z, Qian H et al,. Novel fatty chain-modified glucagon-like peptide-1 conjugates with enhanced stability and prolonged in vivo activity (2013) Biochem Pharmacol 86: 297-308.
https://doi.org/10.1016/j.bcp.2013.05.012
9. Okhay N, Jegat C, Mignard N and Taha M. PMMA thermoreversible networks by diels-alder reaction (2013) Reactive and Functional Polymers 73: 745-755. https://doi.org/10.1016/j.reactfunctpolym.2013.02.006
10. Ye R, Xiang C, Lin J, Peng Z, Huang K et al,. Coal as an abundant source of graphene quantum dots (2013) Nature Communications 4: 2943.
https://doi.org/10.1038/ncomms3943
11. Li J, Vergne MJ, Mowles ED, Zhong WH, Hercules DM, et al. Surface functionalization and characterization of graphitic carbon nanofibers (GCNFs) (2005) Carbon 43: 2883-2893. https://doi.org/10.1016/j.carbon.2005.06.003
12. Dubois J, Melwin CM, Rondelet G and Wouters J. Synthesis and crystallographic characterization of a maleimide derivative of tryptamine (2016) Crystals 6: 153. https://doi.org/10.3390/cryst6110153
13. Johra FT, Lee JW and Jung WG. Facile and safe graphene preparation on solution based platform (2014) J Ind Eng Chem 20: 2883-2887.
14. Li X, Bandyopadhyay P, Nguyen Th, Park O and Lee J. Fabrication of functionalized graphene oxide/maleic anhydride grafted polypropylene composite film with excellent gas barrier and anticorrosion properties(2018) J Membrane Sci 547: 80-92.
https://doi.org/10.1016/j.memsci.2017.10.031
15. Thangavel P, Kannan R, Ramachandran B, Moorthy G, Suguna L, et al. Development of reduced graphene oxide (rGO)-isabgol nanocomposite dressings for enhanced vascularization and accelerated wound healing in normal and diabetic rats (2018) Journal of Colloid and Interface Sci 517: 251-264. https://doi.org/10.1016/j.jcis.2018.01.110
16. Luo Ch, Zhou L, Chiou K and Huang J. Multifunctional graphene hair dye (2018) Chem 4: 784-794.
https://doi.org/10.1016/j.chempr.2018.02.021
17. Zakeri M, Abouzari-lotf E, Miyake M, Mehdipour-Ataei and Shameli K. Phosphoric acid functionalized graphene oxide: A highly dispersible carbon-based nanocatalyst for the green synthesis of bio-active pyrazoles (2019) Arabian J Chem 12:188-197.
https://doi.org/10.1016/j.arabjc.2017.11.006
18. Huq R, Samuel E, Sikkema W, Nilewski, Lee Th et al. Preferential uptake of antioxidant carbon nanoparticles by T lymphocytes for immunomodulation (2016) Scientific Reports 6: 33808.
*Corresponding author
Grigoriy Sereda, Department of Chemistry, University of South Dakota, 414 E. Clark St., Vermillion, SD 57069, United States, E-mail: gsereda@usd.edu
Citation
Sereda G, Sarder R and Keppen J. Mechanochemical organic functionalization of graphite produces tunable coatings of carbon fibers by multilayered graphite microparticles (2019) Nanomaterial Chem Technol 1: 23-31
Keywords
Mechanoactivation, Synthesis, Microparticles, Carbon fibers, Graphite


 PDF
PDF