Research Article :
Grigoriy Sereda*, Roman Sarder and Joseph Keppen The properties of carbon fiber reinforced composites depend on the adhesion of fibers to the polymer matrix. The improvement of interfacial properties in carbon fiber containing composite materials was extensively studied by introducing water-soluble derivative of graphene, graphene oxide and linkers molecules that were dispersed in the fiber sizing onto the surface of individual carbon fibers. Here we report mechano-chemical synthesis of a series of functionalized multilayer graphite micro particles able to modify carbon fibers simply dipped to their suspensions in various solvents, including water. The known mechanoactivated chemical exfoliation of graphite by maleic anhydride and maleimide was expanded to hexadecylmaleimide and 1,6-dimaleimidohexane. The new materials were characterized by SEM imaging, X-ray Photoelectron Spectroscopy (XPS), Fourier Transform Infrared Spectroscopy (FTIR), powder X-Ray Diffractometry (XRD), nitrogen adsorption isotherm surface analysis, and Diffuse Light Scattering (DLS). The coatings produced on both sized and unsized carbon fibers are resistant to ultrasonication. The pH-dependent dispersibility in water and particle size, relatively high surface area, presence of the thiol-reactive maleimide group in the material cross-linked with 1,6-dimaleimidohexane are the welcome properties for drug delivery applications. The facile introduction of various groups to carbon fibers will help tune their properties for industrial applications. Four novel functionalized microparticles
of graphite have been prepared directly from graphite
by the modified approach of mechanoactivated reactive exfoliation or by
refunctionalization. Carbon fibers are known to increase strength of polymer
composite materials, especially for the applications where high rigidity and
low weight are of value. The interface between carbon fibers and the polymer
matrix that surrounds those plays an important role in the material properties
of the composite
material [1,2]. the low affinity of most plastic
matrices to the crystalline graphitic domains of carbon fibers is usually
remediated by coating the fibers with sizing-a polymer with the functional
groups improving adhesion. A better control over the surface
of carbon and greater versatility of its properties can be achieved by the
direct introduction of functional groups. While covalent modification of low
reactive graphitic basal planes is difficult, their ability for π-π stacking
can be utilized for direct functionalization. Thus, the pyrene moiety was used
as an anchor for introduction of N-hydroxysuccinimide or gold
nanoparticles to carbon nanotubes [3,4]. The
availability of the graphitic basal planes in the edge-functionalized particles
of graphene makes them a promising material for rapid and versatile
functionalization of carbon fibers tailored to the polymer matrix in the
desired composite. Considering the known improvement of the mechanical
performance of carbon fiber/epoxy composites by grafting graphene oxide into
the fibers, development of versatile functionalized graphenes presents a
significant practical interest [5]. Besides of a rather expensive
synthesis of graphene by the chemical vapor deposition, a variety of top-down
methods exist as well. Graphite
oxidation and subsequent reduction produce a
material called reduced graphene oxide, which is a graphene with more defects
[6,7]. In 2013, Baek and coauthors have
reported a method that exfoliates graphite into graphene and adds
functionalization at the same time due to the Diels-Alder addition of maleic
anhydride or maleimide to the diene moieties of the mechanoactivated graphitic
layers [7]. While Seo
JM, et al., refers to the particles as graphene,
their structure seems to better fit the category of micro particles of graphite
[7]. This solvent-free mechanochemical procedure produces multilayered graphite
microparticles functionalized at the edge of their graphitic planes at the gram
scale, and requires inexpensive reagents and a ball mill. However, only two
functional groups (anhydride and imido-group) have been introduced to the micro
particles. Here we report facile preparation
of three new types of edge-functionalized graphite micro particles and their
adhesion to unsized and sized industrial carbon fibers along with a series of
existing carbonaceous materials. The hexadecylimido- and
1,6-hexane-bis-imido-functionalized micro particles of graphite were prepared
by the expansion of the mechanoactivated chemical exfoliation of graphite to
the N-hexadecylmaleimide
and 1,6-dimaleimidehexane dienophiles. The
(2Z)-4-(6-aminohexyl-1-amino)-4-oxobut-2-enoic acid functional group was
introduced to the anhydride-functionalized micro particles of graphite by their
reaction with 1, 6-diaminohexane. Materials and
equipment 1-Hexadecylamine, triethylamine
and diisopropyl carbodiimide (DIC)from Alfa Aesar, graphite powder with a
diameter of<20 µm - from Sigma-Aldrich, Fmoc-6-aminohexanoic acid
(Fmoc-Ahx-OH) - from Chem-Impex International, maleic anhydride, acetic
anhydride, nickel (ll) acetate, acetic acid, hexane, methanol, N-hydroxybenzotriazole
(HOBt), maleimide, N,N-dimethylformamide
(DMF), 1,6-diaminohexane and dichloromethane (DCM) -from Oakwood Chemicals.
Graphene: Unmodified GraphenX™ graphene was purchased from www.cheaptubes.com
and used as-is without further modification. The carbon fibers of the SGL APS
type were provided by SGL Corporation. XRD analysis was carried out using a
monochro XRD instrument with energy of 40.0 kV by Rigaku Ultima IV. SEM measurements
were carried out using InLense and SE2 detector with an energy of 1.50 to 3.00
kV by SIGMA ZEISS. FTIR measurements were carried out from 400 to 4000 cm-1
by Perkin Elmer. The ball millings for graphite
functionalization were carried out by Planetary
Ball Mill (PBM-04, Micro Nano Tools). XPS measurements were carried out using
monochromatic Al Ka x-ray with energy of 1486.6 eV by Thermo Scientific K-alpha+
XPS. The hydrodynamic particle sizes have been analyzed by the DLS (Dynamic
Light Scattering) method with Zetasizer (model: Malvern Zetasizer Nano Series).
Ultrasonication was performed in a Fisher Scientific FS20H ultrasonic bath.
UV-vis absorption spectra were measured on Varian Cary 50 UV-Vis spectrophotometer.
Braunauer-Emmett-Teller (BET) analysis for the determination of surface area
was done by Quantachrome Novawin (version 11.03). 1H NMR spectra
were recorded on a Brucker 400 MHz spectrometer in CDCl3 and TMS
(tetramethylsylane) as an internal standard. Synthesis of
dienophiles N-hexadecylmaleimide was prepared
by a modified known procedure [8]. Briefly, 1-Hexadecylamine (22.99 g, 0.095
mol) and maleic anhydride (10.51 g, 0.107 mol) were refluxed in 150 mL glacial
acetic acid until completion (approximately 6 h, monitored by 1H
NMR). The reaction mixture was allowed to cool to room temperature and poured
into 2000 mL of cold deionized water with stirring. This mixture was stirred
for 6 h and filtered through filter paper. The solid material is dried in air,
broken into a powder, washed repeatedly with deionized water to remove residual
acetic acid, and dried in a vacuum desiccator. Yield 28.9 g (84.0%). M.p.
45-55°C; lit. M.p. 103-105°C. 1H NMR (CDCl3, 400 MHz): δ
6.67 (s, 2H), δ 3.50 (t, 2H, J= 7.2 Hz), δ 1.63 (m, 28H, 7.56), δ 0.87 (t, 3H).
By NMR, the purity of the compound is estimated as ~96%. The product was used
without further purification [8]. 1,6-Bismaleimidohexane
was prepared by a modified known procedure. briefly, maleic anhydride (49.0 g,
0.5 mol) was dissolved in 150 mL DMF in a 500 mL round bottom flask at magnetic
stirring. Next, 1,6-diaminohexane (29.05 g, 0.25 mol) was added to the flask at
stirring. The solution slightly heated up and turned translucent yellow [9]. The temperature was maintained at
90°C for 30 min on a hot plate. Then acetic anhydride (94 mL, 0.99 mol),
triethylamine (13.8 mL, 0.099 mol), and nickel (II) acetate tetra hydrate (0.5
g, 0.0028 mol) were added within 5 min. After the temperature was maintained
for 30 min, the reaction mixture was allowed to cool to 40° C and was poured to
2L of deionized water in an ice bath. The brown precipitate was filtered,
washed with 1L of water in small portions, dried at 80°C in air for 3 days. The
solid mass was crushed into a powder, stirred with 2L of water, filtered, and dried
at 80°C in air for 4 h. Yield 120.9g (88%). MP. 125-130°C, lit. m.p. 144°C. 1H
NMR (CDCl3, 400 MHz): δ 6.70 (s, 4H), δ 3.52 (t, 4H, J=7.4 Hz), δ
1.59 (m, 4H), δ 1.32 (m, 4H). By NMR, the purity of the compound is estimated
as ~95 % [9]. Preparation of graphite
micro particles
In a typical procedure, 3 g of
graphite and 6 g of a dienophile
(maleic anhydride, maleimide, N-hexadecylmaleimide or 1,6-dimaleimidohexane)
were ground in a 50 mL stainless steel jar using a planetary ball mill
operating at 500 rpm for 48 h [7]. The reaction mixture was washed with a
solvent (hexane for N-hexadecylmaleimide, methanol for maleimide, methanol for
Bis-maleimide, methanol for maleic anhydride) on a fritted glass funnel. The
graphite micro particles were dried, weighed, and washed again. The washing
cycles continued until the constant weight of the product (typically, 3-6 mL of
each solvent was used in each of 3 washing cycles). The extent of
functionalization was estimated by the gravimetrically measured weight gain [7]. Non-functionalized
graphite micro particles: Graphite was processed in the
same ball mill under the same conditions but without a dienophiles to produce
the control material GC. Pre-treatment
and non-covalent modification of carbon fibers:
Unsized carbon fibers were prepared by heating chopped 1-2 bundles of sized
fibers (APS) in a nitrogen purged furnace at 500°C for 5 h to remove any
surface coating. The fibers to be modified (100 mg) were sonicated in a
suspension of 100 mg of graphite micro particles in 10 mL of a solvent for 3
min, filtered and washed with 5 mL of the same solvent. Preparation of
graphene quantum dots These particles were made using a
modified method based on Ye, R et al. Briefly, a 300 mg portion of anthracite
coal was ultrasonicated in 60 mL of 96% H2SO4 and 20 mL
60% HNO3 for 6 h, then heated to 100°C for 24 h with continuous
stirring. After cooling to room temperature, the solution was poured over 100 g
of ice. The pH of the solution was brought to ~7 by a 3M (and at the end –
0.01M) sodium hydroxide solution. The solution was decanted, passed through a
0.45 µm PTFE syringe filter, dialyzed through a 3500 Da membrane against
deionized water for 7 days. The solvent was removed in vacuum to produce the
material GQD [10]. Preparation of
graphite micro particles functionalized with
(2Z)-4-(6-aminohexyl-1-amino)-4-oxobut-2-enoic acid (GAM) A mixture of 3 g of maleic
anhydride functionalized graphene (GMA) and 33 g
of 1,6-diaminohexane was stirred in 100 ml of DMF at 110oC for 20
min, next at 90oC for 2h. The mixture was cooled to room temperature
and centrifuged. The precipitate was washed with DMF (3X13 mL) and dried at
room temperature in air, yield 84% (GAM). Determination
of amino-groups on carbon The density of amino-groups was
calculated using the following equation [11]: Density of NH2 (mmol/g) = Absorbance *Dilution Factor (DF) Mass of the material * 1.75 DLS
measurements: A 5 mg sample of a material was
suspended in 10 mL of deionized water by ultra-sonication for 10 min, decanted,
and measured on the Zetasizer instrument. XRD analysis A 4-6 mg of a sample was placed
on the sample holder and measured from 5 to 80 degree with a scan speed of
0.300 deg/min and sample width of 0.0200 degree. The data was processed by
PDXL2 software. SEM imaging A few drops of a suspension of ~5
mg of a sample in 10 mL of hexane was affixed on a silicon wafer with a carbon
double sticky tape and dried on air. The sample was rinsed with hexane and
dried on air again. FTIR analysis A 3-5 mg of a sample was ground
in a mortar with ~50 mg of dry KBr and pressed into transparent KBr pellets.
The transmission data in the 400-4000 cm-1 range was processed by Spectrum2
software. XPS analysis
A 2-5 mg sample was attached to a
conductive stage by double-sided carbon tape and held at 5*10-8
mbar for 4 h before the experiment. The data were processed by the Advantage
software package. Surface analysis
An approximately 0.05 g sample of
a material was evacuated and pre-heated at 100oC for 5.5 to 14 h.
Next, the sample was cooled by liquid N2, and the adsorption of N2
gas was measured at an array of pressures. The surface area was calculated by
the Brunauer-Emmett-Teller (BET) analysis, and the Barrett, Joyner and Halenda
(BJH) method was used to calculate the average pore size and volume. Functionalized
graphite microparticles Preparation
of functionalized graphite microparticles by ball milling with a dienophile: The
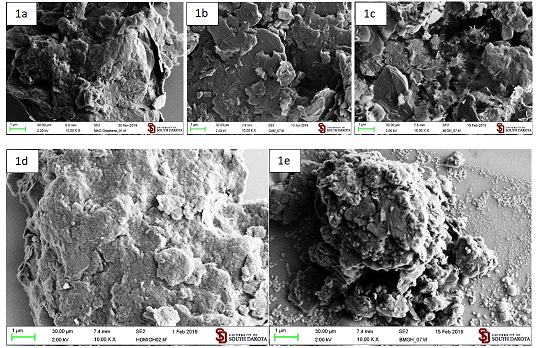
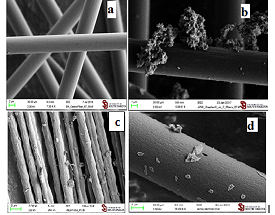
graphite microparticles functionalized with maleimide (GMI, Figure 1b) were
prepared in air with maleimide as the dienophile, which produced 4.5% weight
gain. The extent of functionalization depended on the reaction conditions. When
the ball milling was performed in air, the weight gain was 4.5%, increased when
the reaction was run under nitrogen (16% weight gain) and reached 27% under 74
millitorr vacuum. The SEM analysis did not reveal any substantial influence of
the reaction atmosphere on the size and morphology of graphite microparticles. All
other functionalized microparticles of graphite have been prepared under vacuum
to minimize potential oxidation by the atmospheric and adsorbed air. The graphite microparticles
functionalized with maleic anhydride (GMA, Figure
1a) were prepared under 80 millitorr vaccum with maleic anhydride as the
dienophile, which produced 7.2% weight gain. The graphite microparticles
functionalized with N-hexadecylmaleimide (GHDMI, Figure 1c) were prepared under 84 millitorr vacuum conditions with
N-hexadecylmaleimide as the dienophile, which produced 5.4% weight gain. The graphite microparticles
functionalized with 1,6
dimaleimidohexane (Gbis, Figure 1d) were prepared under 80 millitorr vacuum (weight gain
6.2%) or under nitrogen (weight gain 13%) with 1,6-dimaleimidohexane as the
dienophile. Thus, we optimized the conditions of the mechanochemical synthesis
and expanded its applicability to N-substituted maleimide. Graphite
microparticles functionalized with (2Z)-4-(6-aminohexyl-1-amino)-4-oxobut-2-enoic
acid (GAM) This aminated material was
prepared by re-functionalization of the maleic anhydride derivative GMA with
excess of 1,6-diaminohexane. SEM images of functionalized graphite
microparticles prepared by Mechanoactivation (a-d) are presented in Figure 1. The
direct quantitative analysis of amino-groups on carbons is difficult because
carbon absorbs and catalyzes degradation of most of the dyes involved in the
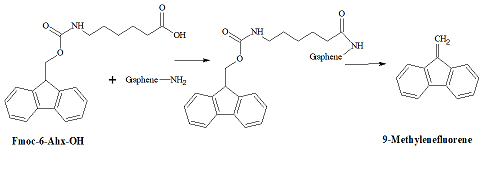
analysis. Therefore, we employed the Fmoc-based analytical protocol [11] (Figure 2). Figure 2: Analysis for the
surface amino groups. First,
the amino-groups on the carbons surface are covalently coupled with a
chromophore-containing Fmoc-6-Ahx-OH. Next, after removal the excess of
Fmoc-6-Ahx-OH, cleavage of the Fmoc-moiety with piperidine releases into
solution one molecule of 9-methylenefluorene per each surface amino-group,
which is determined photo metrically. In the aminated graphite microparticles
GAM, the density of amino-groups determined by the Fmoc method was 0.22 m mol/g
[11]. The
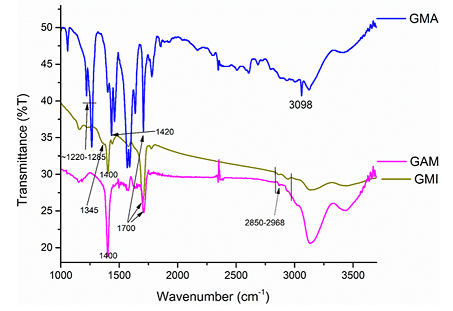
surface functional groups have been identified by FT-IR spectroscopy. The
strong adsorption at ~1700 cm-1 is characteristic to the stretching
C=O vibration in the material functionalized with imido-groups (GMI), the
material functionalized with maleic anhydride (GMA), and its derivative open
with a diamine (GAM) (Figure 3). For
the maleic anhydride derivative (GMA), the C=O vibration occurs below the
characteristic for the anhydride 1800 cm-1 wavenumber. These points
to the hydrolysis of the anhydride ring, which is consistent with the literature
[7]. The presence of the absorption band at ~3098 cm-1, which is
characteristic for C(sp2)-H stretching vibrations indicates that
some molecules of maleic anhydride acylated OH- groups available at the
oxidized edges of graphene layers. The absence of
an unreacted phase of maleic anhydride was confirmed by the powder XRD
analysis. No multiple reflections characteristic for maleic anhydride were
observed [12]. The materials functionalized with weak acylating agents - maleimide
derivatives did not show the C (sp2)-H band. A series of bands at
~1200-1285 cm-1 characteristic for the presence C-O bonds is
especially strong for the maleic anhydride derivative. The nitrogen-containing
GMI shows a weak shoulder at ~1345 cm-1 due to C-N absorption, which
is barely visible in GAM. The strong ~1580-1600 cm-1 bands of the
stretching vibrations of non-symmetrical C=C in GMA are much weaker in the
nitrogen-containing materials GMI and GAM and almost not visible in the
materials GHDMI and Gbis (Figures 3 and
4). Introduction
of sp3-carbons to the materials in the processes of cycloaddition
and a diamine acylation leads to the C-H stretching vibrations at ~2850-2968 cm-1.
Broad absorptions at ~3000-3500 cm-1 characteristic for -OH and -NH
stretching vibrations are especially strong for the aminated material (GAM) (Figure 3). Figure 3: FTIR-spectra of
known (GMA, GMI) functionalized graphite microparticles and the aminated
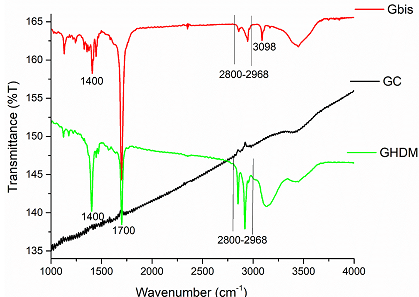
material (GAM). Figure 4: FTIR-spectra of
new functionalized graphite microparticles prepared directly by
Mechanoactivation (GHDMI, Gbis). The ball milled non-functionalized graphite
(GC) is a reference. The
absorption patterns of new functionalized graphite microparticles directly
synthesized by ball milling of graphite with long-chain derivatives of
maleimide are similar to the materials GMA, GMI and GAM. Due to the presence of
long alkyl chains, the new materials show prominent absorptions at ~2850-2968
cm-1, which is especially strong for the GHDMI derivative,
containing hexadecyl groups (Figure 4).
The material Gbis functionalized with a molecule with two maleimide moieties
shows an absorption band at ~3098 cm-1, which is characteristic for
C (sp2)-H stretching vibrations. These points to the presence of
free maleimide groups in the graphite microparticles, which may enable their
subsequent cross-linking and re-functionalization. According
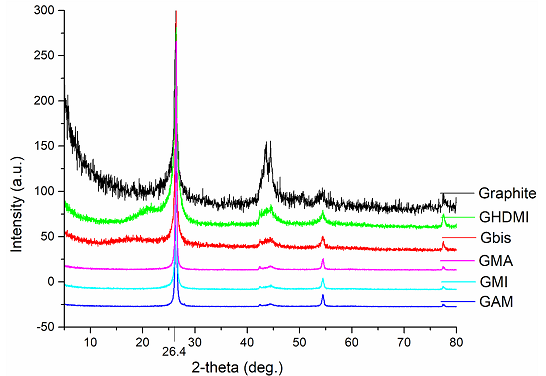
to the XRD diffraction patterns of the functionalized graphite microparticles,
the spacing between graphene layers (~3.5Å, 2θ=26.4o) matches the
one in the parent graphite, which is consistent with their multilayer morphology. This allowed us
to categorize the particles as multilayered particles of graphite. This lack of
other reflections confirms that the material does not contain any maleic
anhydride impurity, which is known to produce a multitude of reflections scattered
all over the spectral range [12,13] (Figure
5). Figure 5: XRD patterns of
functionalized multilayered graphene microparticles. Surface analysis The
microparticles of Gbis (more projections are given in Figure 6a and Figure 6b preserve the high porosity of the material
and their morphology is very similar to that of the control material GC (ball
milling without a dienophile) (Figure
6). Figure 6: SEM micrographs
of a, b Gbis microparticles. c. Control material GC. This
is in contrast with all other low porous functionalized materials (Figure 1). The retention of the
morphology in Gbis could be attributed to the cross-linking ability of
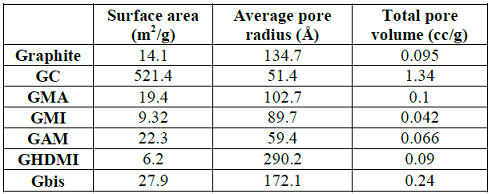
1,6-dimaleimidohexane. The surface analysis results of the new materials are
presented in Table 1. Table 1: Surface analysis
of functionalized graphite microparticles. The
kinetic energy generated in the mill breaks graphite chunks into small
particles, which drastically increases the surface area and the total pore
volume. However, the resulting GC material is not functionalized, and its pores
are significantly shrunk comparing with graphite. The increase of the surface
area of graphite upon mechanoactivated functionalization is much less
pronounced while functionalization with HDMI (N-Hexadecylmaleimide) even
decreases this parameter. Among all functionalized microparticles of graphite,
the Gbis material has the highest surface area and total pore volume, perhaps,
due to the cross-linking by the bifunctional Bis-maleimide. The drastic
increase of solvation of Gbis by water and methanol compared with even more
polar GMA and GAM further points at the unique properties of the cross-linked
Gbis, which contains both hydrophobic alkyl chains and reactive
maleimide-groups while maintaining the pore size of the bulk graphite. The
type of the functional group on the graphite microparticles significantly
affected their dispersibility in DCM, methanol, and water, which was estimated
by sonicating 10 mg of the microparticles in 5 mL of the solvent for 6 min and
observing the suspension for 1 week. The
control material GC (ball mill grounded graphite microparticles) was suspended
to test their stability in different solvent systems. The suspended GC was
mostly stable in water up to 3 days while in hexane it completely precipitated
after 40 minutes, and in DCM it took 60 minutes for complete precipitation. The
microparticles MA and MI prepared by the known method did not form a stable
suspension with water, hexane, or DCM. However, relatively stable suspensions
(not completely separated after 1 day) were obtained in methanol. The suspension
of control GC in methanol was stable up to 3 days [1]. The
most hydrophobic GHDMI containing long alkyl chains (16 carbons), expectedly
failed to form a suspension in water. It is suspension in methanol was stable
for 1 day and completely separated in 1 week. GHDMI was best solvated by DCM,
which produced a suspension still stable after 1 week. The least polar hexane,
however, did not sustain a stable suspension with neither GHDMI nor any other
functionalized microparticles. Therefore, introduction of a long alkyl chain to
a maleimide-functionalized microparticle of graphite significantly increased
its dispersibility in methanol, and especially – in DCM. In
the material Gbis, the maleimide moieties are cross-linked with a short alkyl
chain (6 carbons). The suspension of Gbis in DCM was less stable than that of
GHDMI: while persisting for 1 day, it completely separated in 1 week. However, dispersibility
of Gbis in methanol noticeably improved compared with GHDMI: the Gbis
suspension in methanol still persisted after 1 week. Remarkably, Gbis was the
only type of functionalized microparticles that was able to form an aqueous
suspension still stable after 1 day. Although the suspension completely
separated in 1 week, its stability was sufficient for the industrial
modification of carbon fibers using a green non-toxic and non-flammable
dispersant. The drastic increase of solvation of Gbis by water and methanol
compared with even more polar GMA and GMI is consistent with the higher
porosity of Gbis and the cross-linking ability of 1,6-dimaleimideohexane. Due
to the presence of acidic groups on the graphitic surface, the stability of the
aqueous suspensions was higher at higher pH values, adjusted by aqueous HCl and
NaOH. While after 7 h, the aqueous suspension of Gbis at pH = 3 completely
separated, the particles mostly remained suspended at pH = 7 to 11. The
observed dispersibility of the materials was consistent with the pH-dependent
particle sizes measured by DLS. Thus, changing pH from 7.0 to 5.0 led to the
substantial increase of the average particle size from 367 to 526 nm. This
makes the Gbis material a candidate for a pH-controlled drug delivery scaffold (Table 2). Table 2: pH-Effect on the
size of Gbis particles determined by DLS. Modification of
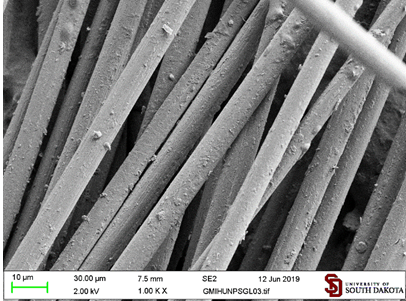
carbon fibers with known carbon particles For
comparison, we examined adhesion of four types of carbon particles (graphite,
commercial GraphenX, carbon quantum dots GQD,and graphite milled
without a dienophile (GC)) to carbon fibers [10] (Figures 6c and 7). Figure 7: Carbon
particles: (a) unmodified graphite, (b) Graphene™, (c) Graphene quantum dots
GQD. The
treatment of carbon fibers with an aqueous suspension of carbon quantum dots (GQD)
led to a flat positioning of the dots over the surface of fibers (Figure 8d),
while the commercial graphene (GraphenX) formed clusters just loosely
associated with the carbon fibers surface (Figure 8b).
Commercial graphite did not coat carbon fibers from its aqueous suspension at
all (Figure 8c). However, it did deposit loosely associated clusters from its
suspension in hexane (Figure 8). Figure 8: a. untreated
carbon fibers. Carbon fibers treated with suspensions of: b. GraphenX in water;
c. Graphite in hexane; d. Carbon quantum dots GQD in water. The
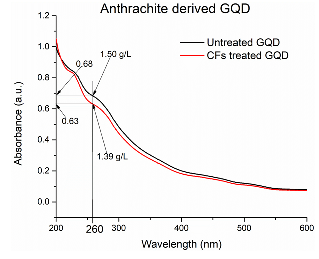
deposition of quantum dots on carbon fibers was additionally confirmed by their
reduced concentration after exposure to the fibers. An aqueous suspension of
carbon quantum dots was divided into two parts. After one part (30 mL) was
exposed to carbon fibers (100 mg), concentration of carbon quantum dots was measured in
both parts by UV-vis spectroscopy (Figure
9). Concentration
of GQD in the stock solution measured by complete evaporation of the solvent
was 1.50 g/L. The concentration of GQD in the solution after exposure to carbon
fibers was estimated by the Beer-Lambert Law at 260 nm as 1.39 g/L. Therefore,
the amount of GQD deposited on 0.1 g of carbon fibers was (1.50 – 1.39) g/L X
0.030 L = 0.0033 g, which 3.3 wt%. Figure 9:
UV-vis-absorption by aqueous suspension of carbon quantum dots. Blue line–before
exposure to carbon fibers. Red line– after exposure to carbon fibers. Modification of
carbon fibers with graphite microparticles functionalized with maleic anhydride Maleic
anhydride functionalized graphite microparticles (GMA) coat carbon fibers by a simple dipping to their suspension in
hexane (Figure 10). Figure 10: Carbon fibers
coated with graphite nanoparticles functionalized with maleic anhydride (GMA)
in hexane. Modification of
carbon fibers with graphite microparticles functionalized with maleimide The
coating obtained from the maleimide functionalized graphite microparticles (GMI) seems to
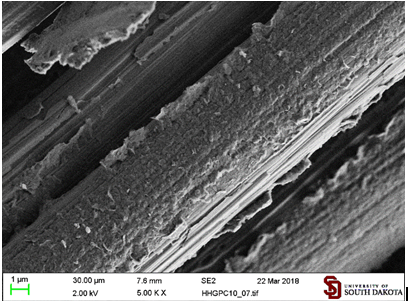
be denser and with fewer particles sitting on their edges (Figure 11). Modification of
carbon fibers with graphite microparticles functionalized with
hexadecylmaleimide The
coating by hexadecylmaleimide functionalized graphite microparticles (GHDMI)
consists of long flakes almost completely covering the surface of fibers (Figure 12). Figure 12: Carbon fibers
coated by graphite microparticles functionalized with hexadecylmaleimide (GHDMI) from
their hexane suspension. Introduction
of the GHDMI material to the surface of carbon fibers significantly affects its
XPS spectrum. The surface elemental analysis of commercial carbon fibers
reveals the presence of a noticeable amount of oxygen (C-86.08%, N-3.84%,
O-10.08%) due to the plasma treatment routinely used to improve the adherence
of fibers to the sizing (coating of carbon fibers before placing them to a
composite material). The GHDMI – coated surface contains less oxygen due to the
blockage by the long alkyl chains and more carbon because of the high carbon
content of alkanes (C-89.98%, N-3.12%, O-6.91%). Modification of
carbon fibers with graphite microparticles functionalized with 1,6-dimaleimidohexane. The
treatment of carbon fibers with a hexane suspension of graphite functionalized
with 1,6-dimaleimidohexane (Gbis) produced a dense coating of the fibers with
the material (Figure 13). Figure 13: Carbon fibers
coated by graphite microparticles functionalized with 1,6-dimaleimidohexane
(Gbis) from their hexane suspension. The
strongest effect of Gbis on the
elemental composition of the surface is substantial increase of the nitrogen
content due to the presence of two atoms of nitrogen in the
1,6-dimaleimidehexane linker (C-85.81%, N-4.92%, O-9.27%). The chemical
environment around the atoms of carbon and oxygen on the surface of modified
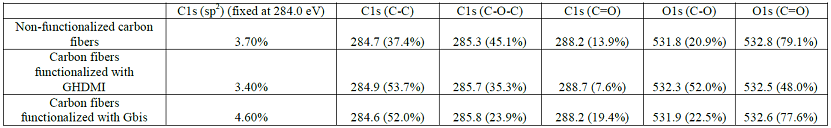
carbon fibers was examined by the deconvolution analysis of the 1s bands
with the sp2 component fixed at 284.0 eV (Table 3). The
long alkyls chains in GHDMI introduce to the surface more C-C bonds while the
highly oxidized C=O groups are blocked by the adsorption of the hydrophobic
graphite particles, which is evident from the chemical environment changes
around both carbons and oxygens. This
shifts the balance of the oxygen chemical environment from C=O to C-O groups. Similarly
to GHDMI, the Gbis particles also introduce the C-C bonds to the surface.
However, the graphene surface of the particles is not blocked by hexadecyl
chain and introduces its own C=O groups to the surface. Thus,
despite the preferential adsorption of the particles on the C=O groups of the
surface of carbon fibers, their contribution to the chemical environment both
around carbons and nitrogens does not substantially decrease. The abundance of
maleimido- in surface C=O groups in Gbis shows in the increased contribution of
the sp2 component. Table 3: Structural XPS
analysis of modified carbon fibers. Modification of
carbon fibers with graphite microparticles functionalized with
(2Z)-4-(6-aminohexyl-1-amino)-4-oxobut-2-enoic acid We
found that the aminated graphite microparticles efficiently adhere to carbon
fibers from their DCM, hexane, or aqueous suspensions. The SEM images on Figure 14 show that both flat and edge
areas of the particles demonstrate substantial affinity to the fibers. Figure 14: Carbon fibers
coated by the aminated graphite microparticles (GAM) suspended in (a) DCM; (b)
Hexane; (c) Water. Modification of
sized carbon fibers with functionalized graphite microparticles Modification of
sized carbon fibers with graphite nanoparticles functionalized with
hexadecylmaleimide: We
found that the adhesive ability of graphite microparticles is not limited to
carbon-carbon p-stacking interaction. The hydrophobic
interaction seems to play a significant role as well. Thus, graphite
microparticlres functionalized with N-hexadecylmaleimide (GHDMI) adheres to
sized (coated with an organic polymer) carbon fibers upon dipping to their
hexane suspension. The subsequent sonication for 10 min knocked off most of
loosely attached particles, while the well-adhered particles were retained (Figure 15). A similar trend was
observed for the suspension of GHDMI in DCM (Fig. 16). Figure 15: Sized carbon
fibers coated by the graphite microparticles functionalized with
N-hexadecylmaleimide (GHDMI) suspended in hexane. (a) After regular sonication
(3 min); (b) After additional 10 min of sonication. Modification of
sized carbon fibers with graphite microparticles functionalized with
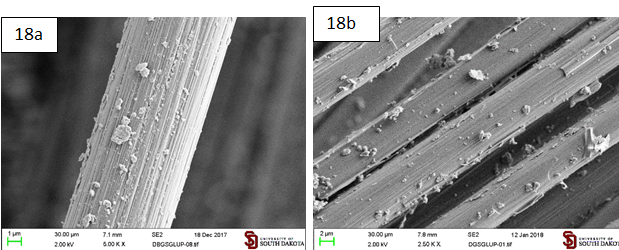
1,6-dimaleimidohexane: Graphite microparticles functionalized with
1,6-dimaleimidohexane (Gbis) adheres to sized carbon fibers from its hexane and
DCM suspensions. This produces a
denser, more evenly distributed and more resistant to sonication coating than GHDMI
(Figures 17 and 18). Figure 17: Sized carbon
fibers coated by the graphite microparticles functionalized with
N-hexadecylmaleimide (Gbis) suspended in hexane. (a) After regular sonication
(3 min); (b) After additional 10 min of sonication. Figure 18: Sized carbon
fibers coated by the graphite microparticles functionalized with
N-hexadecylmaleimide (Gbis) suspended in DCM. a. After regular sonication (3
min); b. After additional 10 min of sonication. The
high Dispersibility of Gbis
particles in water allowed for modification of carbon fibers directly by their
aqueous suspension. This produces a less dense coating than suspensions in
hexane and DCM, which is however resistant to sonication (Figure 19). Figure 19: Sized carbon
fibers coated by the graphite microparticles functionalized with N-hexadecylmaleimide
(Gbis) suspended in water. a. After regular sonication (3 min); b. After
additional 10 min of sonication. Modification of
sized carbon fibers with aminated graphite microparticles: The aminated
graphite microparticles also retained their affinity to sized carbon fibers.
The particles are loosely associating with the fibers mostly by the edges of
particles (Figure 20). Figure 20: Sized carbon
fibers coated by the aminated graphite microparticles (GAM) suspended in (a)
DCM; (b) Hexane; (c) Water. The
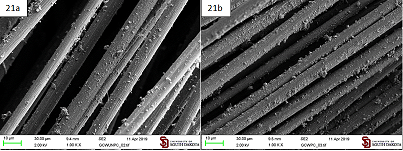
unmodified graphite particles (GC) obtained by simple ball milling of graphite
can evenly coat both carbon fibers and sized carbon fibers by their aqueous
suspension as well as the modified Gbis material. That coating, however, is
lacking the functional groups introduced by functionalized graphite
microparticles (Figure 21). Figure 21: Coating
produced by an aqueous suspension of unmodified graphite microparticles (GC) on
a. Carbon fibers; b. Sized carbon fibers. Thus,
the affinity of functionalized graphite microparticles to carbon fibers is
comparable with the affinity of less available graphene. Further,
functionalization of the particles allows for the fine tuning of their affinity
and other properties, which make them a viable alternative of graphene for many
applications, such as composite films, wound healing, hair dyes, catalysts for
green synthesis, and antioxidants [14-18]. Functionalized
multi-layer graphite microparticles are able to coat both sized and unsized carbon
fibers by simple dipping to their suspensions in various solvents including
water. The produced coatings are resistant to ultrasonication. The ability to
coat carbon fibers depends on both the type of functionalized particles and on the
suspension solvent. Dispersibility of the Gbis material (which contains both
hydrophobic alkyl groups and reactive maleimido-groups) in water and the
particle sizes substantially changes between the pH values 7 and 5, which is
promising in terms of the drug delivery applications. The results of
this work will help efficiently incorporate carbon materials into the composite
materials with a diverse range of applications. The
raw and processed data required to reproduce these findings are available to
download from https://data.mendeley.com/datasets/y5hkynnw6v/1 We
thank the South Dakota Center for Composite and Nanocomposite Advanced
Manufacturing (CNAM), the South Dakota Surface Engineering Research Center
(SERC), the Department of Chemistry of The University of South Dakota, NSF-MRI
grants CHE-1337707 (SEM/EDS instruments) and CHE- 1229035 (400 MHz NMR
spectrometer). NASA EPSCoR grant NNX15AK54A for the financial support of this
work. The
research was performed in part in the Nebraska Nanoscale Facility: National
Nanotechnology Coordinated Infrastructure and the Nebraska Center for Materials
and Nanoscience, which are supported by the National Science Foundation under
Award ECCS: 1542182, and the Nebraska Research Initiative. We also thank Prof.
Jong-Beom Baeks group for providing samples of two functionalized graphenes. References 1.
Ma
Q, Gu Y, Li M, Wang S and Zhang Z. Effects of surface treating methods of
high-strength carbon fibers on interfacial properties of epoxy resin matrix
composite (2016) Applied Surface Sci 379: 199-205. https://doi.org/10.1016/j.apsusc.2016.04.075 2.
Chen
JR, Zhang Y, Wang D and Dai H. Noncovalent sidewall functionalization of
single-walled carbon nanotubes for protein immobilization (2001) J Am Chem Soc
123: 3838-3839. https://doi.org/10.1021/ja010172b 3.
Salice
P, Gambarin A, Daldosso N, Mancin F and Menna E. Noncovalent interaction
between single-walled carbon nanotubes and pyrene-functionalized gold
nanoparticles in water-soluble nanohybrids (2014) The J Phys Chem C 118:
27028-27038. https://doi.org/10.1021/jp505005e 4.
Zhang
X, Fan X, Yan C, Li H, Zhu Y et al. Interfacial microstructure and properties
of carbon fiber composites modified with graphene oxide (2012) ACS Appl Mater
Interfaces 4: 1543-1552. https://doi.org/10.1021/am201757v 5.
Ismach
A, Druzgalski C, Penwell S, Schwartzberg A, Zheng M et al. Direct chemical
vapor deposition of graphene on dielectric surfaces (2010) Nano Lett 10:
1542-1548. https://doi.org/10.1021/nl9037714 6.
Zaaba
NI, Foo KL, Hashim U, Tan SJ, Liu WW et al. Synthesis of graphene oxide using
modified hummers method: solvent influence (2017) Procedia Engineering 184:
469-477. https://doi.org/10.1016/j.proeng.2017.04.118 7.
Seo
JM, Jeon IY and Baek JB. Mechanochemically driven solid-state diels-alder
reaction of graphite into graphene nanoplatelets (2013) Chemical Sci 4: 4273. https://doi.org/10.1039/c3sc51546j 8.
Han
J, Huang X, Sun L, Li Z, Qian H et al,. Novel fatty chain-modified
glucagon-like peptide-1 conjugates with enhanced stability and prolonged in
vivo activity (2013) Biochem Pharmacol 86: 297-308. https://doi.org/10.1016/j.bcp.2013.05.012 9.
Okhay
N, Jegat C, Mignard N and Taha M. PMMA thermoreversible networks by diels-alder
reaction (2013) Reactive and Functional Polymers 73: 745-755. https://doi.org/10.1016/j.reactfunctpolym.2013.02.006 10.
Ye
R, Xiang C, Lin J, Peng Z, Huang K et al,. Coal as an abundant source of
graphene quantum dots (2013) Nature Communications 4: 2943. https://doi.org/10.1038/ncomms3943 11.
Li
J, Vergne MJ, Mowles ED, Zhong WH, Hercules DM, et al. Surface
functionalization and characterization of graphitic carbon nanofibers (GCNFs)
(2005) Carbon 43: 2883-2893. https://doi.org/10.1016/j.carbon.2005.06.003 12.
Dubois
J, Melwin CM, Rondelet G and Wouters J. Synthesis and crystallographic
characterization of a maleimide derivative of tryptamine (2016) Crystals 6: 153.
https://doi.org/10.3390/cryst6110153 13.
Johra
FT, Lee JW and Jung WG. Facile and safe graphene preparation on solution based
platform (2014) J Ind Eng Chem 20: 2883-2887. 14.
Li
X, Bandyopadhyay P, Nguyen Th, Park O and Lee J. Fabrication of functionalized
graphene oxide/maleic anhydride grafted polypropylene composite film with
excellent gas barrier and anticorrosion properties(2018) J Membrane Sci 547:
80-92. https://doi.org/10.1016/j.memsci.2017.10.031 15.
Thangavel
P, Kannan R, Ramachandran B, Moorthy G, Suguna L, et al. Development of reduced
graphene oxide (rGO)-isabgol nanocomposite dressings for enhanced
vascularization and accelerated wound healing in normal and diabetic rats
(2018) Journal of Colloid and Interface Sci 517: 251-264. https://doi.org/10.1016/j.jcis.2018.01.110 16.
Luo
Ch, Zhou L, Chiou K and Huang J. Multifunctional graphene hair dye (2018) Chem
4: 784-794. https://doi.org/10.1016/j.chempr.2018.02.021 17.
Zakeri
M, Abouzari-lotf E, Miyake M, Mehdipour-Ataei and Shameli K. Phosphoric acid
functionalized graphene oxide: A highly dispersible carbon-based nanocatalyst
for the green synthesis of bio-active pyrazoles (2019) Arabian J Chem
12:188-197. https://doi.org/10.1016/j.arabjc.2017.11.006 18.
Huq
R, Samuel E, Sikkema W, Nilewski, Lee Th et al. Preferential uptake of
antioxidant carbon nanoparticles by T lymphocytes for immunomodulation (2016)
Scientific Reports 6: 33808. Grigoriy Sereda, Department of Chemistry, University of South Dakota, 414 E. Clark St., Vermillion, SD 57069, United States, E-mail: gsereda@usd.edu Citation Sereda G, Sarder R and Keppen J. Mechanochemical organic functionalization of graphite produces tunable coatings of carbon fibers by multilayered graphite microparticles (2019) Nanomaterial Chem Technol 1: 23-31 Mechanoactivation, Synthesis, Microparticles, Carbon fibers, GraphiteMechanochemical Organic Functionalization of Graphite Produces Tunable Coatings of Carbon Fibers by Multilayered Graphite Microparticles
Abstract
Full-Text
Introduction
Experimental
Part
It was performed by the modified procedure of determination of amino-groups on carbon
nanofibers. Specifically; 150 mg of the aminated
graphite (GAM), 175 mg (0.5 m mol) of 6-(Fmoc-amino) hexanoic acid (Fmoc-Ahx-OH),
and 67.5 mg (0.5 m mol) of N-hydroxybenzotriazole (HOBt) were mixed in a 15 mL
vial. Then a solution of 80 µL (0.5 m mol) of diisopropylcarbodiimide (DIC) in
1 mL of DMF was added. The mixture was magnetically for 12 h and filtered. The
solid material on the filter was washed with DMF (3 mL), methanol (3 mL), DCM
(3 mL), and dried in air overnight. A 20 mg sample of the dried material was shaken
for 45 min with a 20:80 (v/v) mixture of piperidine and DMF and centrifuged at
10,000 rpm for 4 min. The centrifugate was diluted X20 (dilution factor, DF)
with 20:80 (v/v) mixture of piperidine and DMF, and the absorbance was measured
at 290 nm [11].
Results and
Discussion
FT-IR
Spectroscopy
Powder XRD

Dispersibility
in solvents


Modification of
carbon fibers with functionalized graphite microparticles








Conclusions
Acknowledgements
*Corresponding author
Keywords