Research Article :
Nilesh Pandya, Tejas Gandhi, KR Desai
Growing public awareness about the state of the
environment, chemical product safety and new chemical regulatory policies is
driving demand for leaders who are able to understand the science underlying
environmental challenges and develop innovative solutions. Chalcones belong to
the flavonoid family and display several pharmacological activities which are
very important. They can be used as an initial compound for synthesis of a lot
of compounds. In this research chalcone derivative are made via green chemistry
route and analysed their physical and antimicrobial activity. Introduction Chalcones
(1,3-diaryl-2-propen-1-ones) are flavonoids found in fruits and vegetables,
that attracted attention because of their pharmacological activities such as
antiinflammatory (1-7), antibacterial (8-12), antifungal (13-17), antiviral
(18-22), antioxidant (23-32).antineoplastic (33-41).Near
about 200 years ago the pace of technological change in western society began
to quicken. Wind, water, and animal power, with their limitations of place and
capacity, were supplemented and then replaced by the steam engine, which went
on to power the factories of the industrial revolution. The railroad made it
possible to move things and people quickly over great distances. The telegraph
and, later, the telephone carried communications across the countryside.
Electric lighting supplanted the dim glow of candles, kerosene, and gas lights.
By the beginning of the twentieth century, the notion of progress was closely
linked with technological development, and that linkage intensified in the
following decades. The automobile and the airplane changed not only travel but
the nature of our cities and towns. Radio and then television brought more of
the outside world into everyone’s homes. Knowledge about the causes of diseases
brought new treatments and preventive measures. Computers appeared, and soon
the transistor made them smaller, more powerful, more accessible, and cheaper. Today, the system by which research and
development leads to new products is fundamentally different than it was in the
nineteenth century. To the role of the individual inventor has been added the
power of organized scientific research and technological innovation. Organized
research and development, which are increasingly international in character,
have greatly increased the production of new knowledge. Deeper understanding of
living organisms is leading toward cures of diseases once thought untreatable. Basic insights in materials
science enable the development of structures that are lighter, stronger, and
more durable than anything available before. The computer and novel modes of
communication, such as optical fibers, bring new, interactive modes of work and
more capable machinery. These new devices and new ways of working, in turn,
speed the growth and dissemination of new knowledge. Environmental Protection Over the past two decades, the United
States has recognized and has made substantial progress in curbing the
degradation of the environment. Nevertheless, difficult problems remain. Environmental degradation continues to
accompany many aspects of economic growth. Emissions and effluents of
contaminated substances continue, garbage disposal plagues urban areas, forests
continue to be devastated and biodiversity losses are growing. At
the same time, science and technology have exposed new issues of great
complexity and uncertain consequences, such as global warming, acid
precipitation, the destruction of the stratospheric ozone layer, and the
contamination of water supplies. Many industrial and agricultural practices and
products used today in energy and food production, transportation, and
manufacturing will need to be restructured to prevent pollution if sustainable
economic growth is to be achieved.So by the use of green chemistry to produce a drug via green synthesis
is very important for our society. A relatively new chemical philosophy,
green chemistry is focused on the design and implementation of chemical
technologies, processes, and services that are safe, energy efficient, and
environmentally sustainable. Adopting these innovations gives industry
sustainable product and process alternatives that will continue to meet market
demands while also enhancing sustainability, improving human health, and
driving the economy, thereby advancing the human condition. Twelve
principals of green principals are as under given by Paul Anastas and John Warner. 1. Prevention 2. Atom Economy Synthetic
methods should be designed to maximize the incorporation of all materials used
in the process into the final product. 3. Less Hazardous
Chemical Syntheses wherever
practicable, synthetic methods should be designed to use and generate
substances that possess little or no toxicity to human health and the
environment. Chemical
products should be designed to affect their desired function while minimizing
their toxicity. 5. Safer Solvents and Auxiliaries The
use of auxiliary substances (e.g., solvents, separation agents, etc.) should be
made unnecessary wherever possible and innocuous when used. 6. Design for Energy Efficiency Energy
requirements of chemical processes should be recognized for their environmental
and economic impacts and should be minimized. If possible, synthetic methods
should be conducted at ambient temperature and pressure. 7. Use of Renewable Feedstocks A
raw material or feedstock should be renewable rather than depleting whenever
technically and economically practicable. Unnecessary
derivatization (use of blocking groups, protection/ deprotection, temporary
modification of physical/chemical processes) should be minimized or avoided if
possible, because such steps require additional reagents and can generate
waste. 9. Catalysis Chemical
products should be designed so that at the end of their function they break
down into innocuous degradation products and do not persist in the environment. 11. Real-time analysis for Pollution
Prevention Analytical
methodologies need to be further developed to allow for real-time, in-process
monitoring and control prior to the formation of hazardous substances. 12. Inherently Safer Chemistry for Accident Prevention Figure 1: 6-Bromo-2-phenyl-3-[4-(3-substitutedphenyl-acryloyl)-phenyl]-3H-quinazolin-4-one. Where R = 3-OCH3-4-OH, 4-CH3, 4-Cl, 2-Cl, 2:4-Cl2, 2-OCH3, 4- OCH3, 4-H, 4-N (CH3)2 , 2-OH Experimental →
Preparation of 2-Amino-5-bromo benzoic acid In
a 250 ml R.B.F Anthranilic acid (1.37 g,
0.01 mole) was dissolve in glacial
acetic acid (10 ml , 0.01 mole)and
cooled below 160C. Bromine (0.52 ml, 0.01 mole) was added at 0-5 ºC drop wise to the reaction mixture.
Reaction mixture consisting of the mono- and di-bromo anthranilic acids was
stirred for further 2-3 hours and then boiled up with water (50 ml) containing
concentrated hydrochloric acid (10 ml) and filter when hot with suction. The
insoluble residue was extracted twice more with boiling water. The filtrate
upon cooling yielded abundant precipitate of the 5-bromo anthranilic acid and
insoluble residue consisted of the 3,5-dibromo anthranilic acid. →
Preparation of 6-bromo-2-phenyl-4H-3, 1-benzoxazin-4-one. 2-Amino-5-bromo Benzoic acid (0.01mole) was dissolve in pyridine
(30ml). The solution was cooled and benzoyl chloride (0.02 mole) was added drop wise with constant stirring. After the
addition was complete, the mixture was further stirred for 30 min. at room
temperature. It was then treated with sodium bicarbonate solution (5%) to
remove any unreacted acid. When the effervescences ceased, it was filtered and
washed repeatedly with water in order to remove excess of pyridine. It was
crystallized from dilute ethanol. →
Preparation of 3-(4-acetylphenyl)-6-bromo-2-phenylquinazolin-4(3H)-one. In a 250 ml
R.B.F. mixture of 6-bromo-2-phenyl-4H-3, 1-benzoxazin-4-one (0.01 mole) and p-amino acetophenone (0.01 mole) in dry pyridine (25ml) were refluxed for 10-12 hours
under anhydrous condition, and excess of pyridine was removed under reduced
pressure. The concentrated mass was cooled and poured into ice cold
hydrochloric acid to give a solid product which was filtered and washed with
water till neutral. →
Preparation of 6-Bromo-2-phenyl-3-[4-(3-substitutedphenyl-acryloyl)-phenyl]-3H-quinazolin-4-one. (VIA Conventional
Method) (NP-1 to NP-10) In a 250 ml
R.B.F., 3-(4-acetylphenyl)-6-bromo-2-phenylquinazolin- 4(3H)-one (0.01 mole) in methanol (20
mL) and diff. type of substituted aldehyde (0.01 mole) were taken and to it (5-6 mL) of 5% NaOH solution was added. The reaction mixture was
refluxed for 5-8 hours and then poured into ice water. The solid product was
filtered and washed with water, dried and recrystallised from methanol. →
Preparation of 6-Bromo-2-phenyl-3-[4-(3-substitutedphenyl-
acryloyl)-phenyl]-3H-quinazolin-4-one. (VIA Microwave Method) (NP-1’ to NP-10’) In a 250 ml R.B.F., 3-(4-acetylphenyl)-6-bromo-2-phenylquinazolin- 4(3H)-one (0.01 mole) and diff. type
of substituted aldehyde (0.01 mole)
were taken and to it (5-6 mL) of 5%
NaOH solution was added. The reaction mixture was refluxed for 3-6 Min and then
poured into ice water. The solid product was filtered and washed with water,
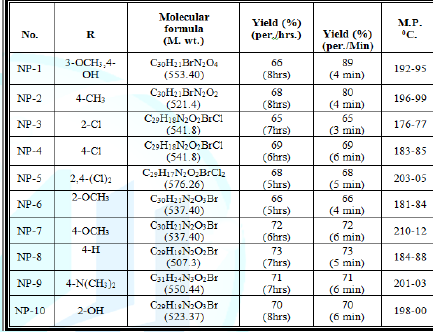
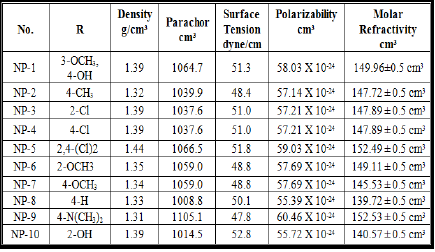
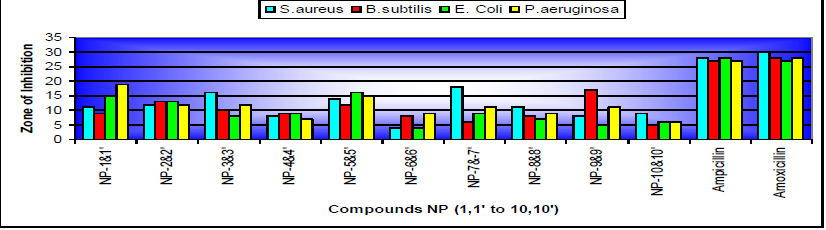
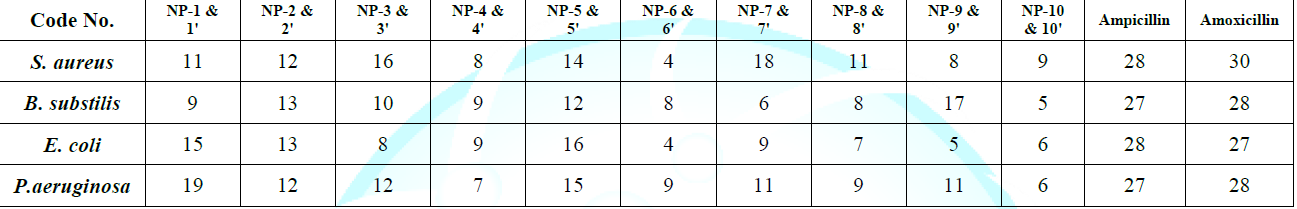
dried and recrystallised from methanol Table 1: Physical and Characterization data of compound. Tables 2: Antimicrobial activity of NP-1 to 10. Table 3: Zones of Inhibition of compounds. References 1. Xia Y, Yang ZY, Xia P, Bastow KF,
Nakanishi Y, et al. Antitumor agents. Part 202: Novel 2¢-amino chalcones:
Design, synthesis and biological evaluation (2000) Bioorg Med ChemLett 10:
699-701. 2. Phrutıvorapongkul A, Lıpıpun V,
Ruangrungsı N, Kırtıkara K, Nıshıkawa K,
et al. Studies on the chemical constituents of stem bark of Millettia
leucantha: isolation of new chalcones with cytotoxic, anti-herpes simplex virus
and anti-inflammatory activities (2003) Chem Pharm Bull 51: 187-190. 3. Bernini R, Mincione E, Coratti A,
Fabrizi G, Battistuzzi G. Epoxidation of chromones and flavonoids in ionic
liquids (2004) Tetrahedron 60: 967-971. 4. Climent MJ, Corma A, Iborra S, Velty A.
Activated hydrotalcites as catalysts for the synthesis of chalcones of
pharmaceutical interest (2004) J Catal 221:474-482. 5. Nielsen SF, Boesen T, Larsen M, Kristian
Schonning K, Kromann H. Antibacterial chalcones-bioisosteric replacement of the
4¢-hydroxy group (2004) Bioorg Med Chem 12: 3047-3054. 6. Lawrence NJ, Mcgown AT. The chemistry
and biology of antimitotic chalcones and related enone systems (2005) Curr
Pharm Des 11: 1679-1693. 7. Nowakowska Z, Kedzia B, Schroeder G.
Synthesis, physicochemical properties and William J. Baumol, Sue Anne Batey
Blackman, and Edward N. Wolff.Productivity and American Leadership: The Long View
(1989) Cambridge, Mass.: MIT Press. 8. Annetine C. Gelijns and Ethan A. Halm,
Eds. The Changing Economics of Medical Technology (1991) National Academy Press,
USA. 9. Carnegie Commission on Science,
Technology, and Government, Task Force on National Security. New Thinking and
American Defense Technology (1990) New York: CarnegieCommission on Science,
Technology, and Government, USA. 10. George Heaton, Robert Repetto, and
Rodney Sobin.TransformingTechnology: An Agenda for Environmentally
SustainableGrowth in the 21st Century (1991) Washington, D.C.: World Resources
Institute USA. 11. Papers from the NAS Colloquium on
Industrial Ecology (1992) Proceedings of the National Academy of Sciences 89: 793–1148. 12. Anastas PT, Warner JC. Green Chemistry:
Theory and Practice (1998) Oxford University Press, USA. Chalcone derivates, Microwave synthesis,Antimicrobial agents.Synthesis of Some Novel Chalcone Derivative Via Microwave Method & Its Antimicrobial Activity
Abstract
Full-Text
It is better to prevent waste than to treat or clean up waste after it has been
created.
Catalytic reagents (as selective as possible) are superior to stoichiometric
reagents.
Substances and the form of a substance used in a chemical process should be
chosen to minimize the potential for chemical accidents, including releases,
explosions, and fires.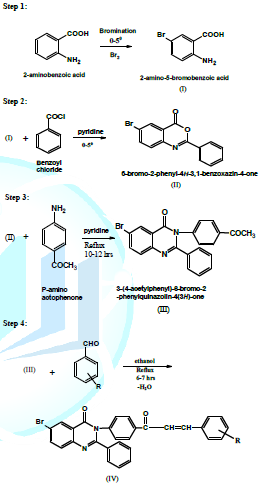
M.P- 219-200C Yield - 80 %
M.P-
205-2070C Yield - 77 %
M.P-
180-890C Yield - 60 %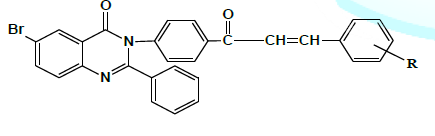
Keywords