Research Article :
Clairmont Griffith and Bernice La France* A
literature review approach is used to explore the impact of opioids on the
human brain. The library directory, internet, and computerswere
essential to the completion of the research. The data was gathered from online
databases including PubMed, EMBASE, and MedLine. The following search terminologies were used: A
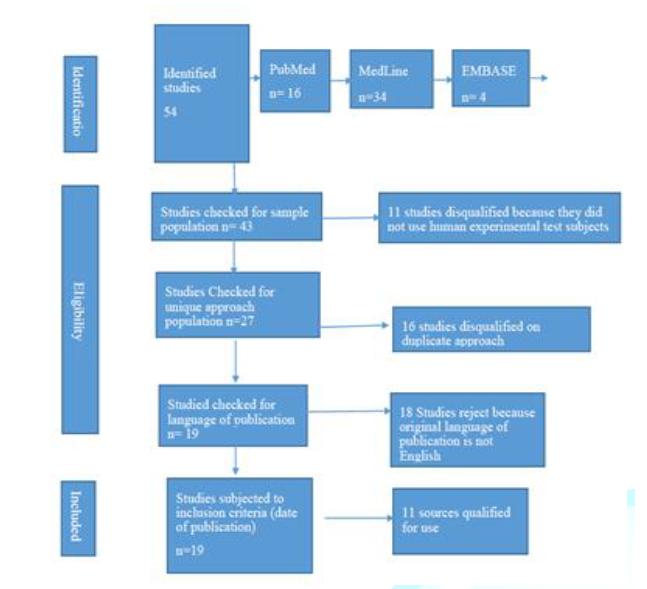
total of 54 secondary sources were identified from the online research. The
sources were scrutinized to determine their relevance and usability in the
study. Sources were eliminated based on sample characteristics. Only sources
based on human subjects were identified for use in the study, 43 studies passed
the test. Only sources that used unique approaches were includes, studies with
duplicate approaches were removed (27 studies qualified). The third criteria
were the language of publication, only studies published in English were used
in the research; 19 studies met the criteria. The search was limited to studies
published from January 2014; 11 articles met the criteria. Moreover, the paper
will be supplemented by discussions on pain management. The discussion below presents the results of
this literature search. Figure 1
outlines the selection criteria. Figure 1: The
selection criteria. Sites of Action
and Inhibition of Neurotransmitters Release Pasternak
& Pan (2013) found that opioids acts on two sites of the brain, the
presynaptic nerve terminal, and the postsynaptic neuron. In the presynaptic
region, opioids have an inhibitory effect on neurotransmitter release; this is
considered its most pronounced effects on the nervous system (Pasternak &
Pan, 2013). However, the summation of opioid action is not limited to its
excitatory and inhibitory effect on the presynaptic region, but also its effect
on the postsynaptic region (Lee, Wanigasekera & Tracey, 2014). For
instance, is the action of opioids causes an inhibitory effect on
neurotransmitter release this may have an excitatory effect if the
neurotransmitter usually causes inhibition in the target neuron (Pasternak
& Pan, 2013). However, if the opiate has an inhibitory effect on the
postsynaptic region, the excitation may not occur. Studies
characterized the impact of opioid on neurotransmitter release by studying the
Ca and K channel. Neurotransmitters release follows depolarization of nerve
terminal Ca++ across the volt-sensitive Ca++ channels (Lamberts & Traynor,
2014). Three types of C++ type channels exist, the L-type, T-type and the
N-type. The release can be inhibited by the increasing outwards K+ release
leading to short polarization time and action potential (Gendron, Cahill, von
Zastrow, Schiller & Pineyro, 2016). This
effect is as a result of the G-protein linking receptors to the K+ channel and
voltage sensitive Ca++ channel, particular, the N-type channel (Lamberts &
Traynor, 2014). However, this effect alone does not completely describe the
effect of Opioids on neurotransmitters. Opioids open the voltage sensitive
potassium ion channels thus increasing outwards flow (Lamberts & Traynor,
2014). The outward movement occurs in several regions of the spinal cords,
brain, and the myenteric plexus. The rapid K+ outward movement is associated
with the hyperpolarization and inhibition resulting from opioids. Opioid
interactions with the adenylate cyclase systems lead to inhibition; the AC is
responsible for the conversion of ATP to Camp. Lamberts & Traynor (2014)
noted that all the three opioid receptors bind to the adenylate cyclase. Location Bias in
Opioids Al-Hasani
& Bruchas (2011) found that morphine has a higher affinity for the
m-receptor than other opioids. While all the three receptors produce an
analgesic effect when bound to opioids, the k-receptor cause a lesser
dependence than m-receptor (Drewes et al., 2012). Both medically used and
natural opioids react with the mu-receptors, a widely occurring protein that
belongs to the GPCRs family. Studies developed a new antibody biosensor to
understand the structure of the GPCRs called a nanobody. The sensor fluoresces
when the GPCR is activated. For naturally occurring opioids, the surface
mu-receptors are activated, and the receptor molecules enter the endosome and
receptor remains active for several minutes (Drewes et al., 2012). However, for
opioid drugs, there is a unique rapid induction of nanobody signaling (in the
range of tens of seconds) in the Golgi apparatus present in the main body the
neuron (Drewes et al., 2012). Activation of Golgi outpost located in the
branched structure was also observed (Lamberts & Traynor, 2014). The
researcher concluded that opioid drugs distort normal spatial sequence and time
of mu-receptor signaling. Pain Modulation Lee,
Wanigasekera & Tracey (2014) studied the effect of opioids on the central
and peripheral nervous system. The study noted that there are critical spinal
and supraspinal opioid-mediated activities leading to a descending pathway. A
study by Garland, Froeliger, Zeidan, Partin & Howard (2013) studied the
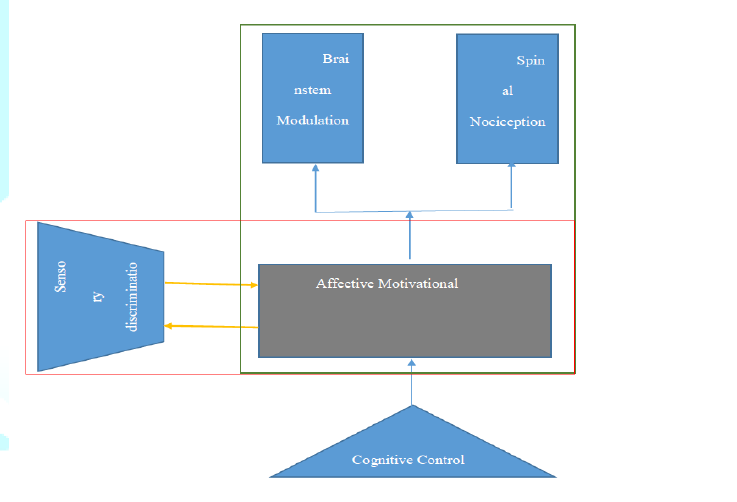
analgesic effect opioids. As illustrated in the figure below, pain perception
results from neural activation in the interconnected brain region shown by the
yellow arrows; these regions have sensory-discrimination and effective
motivation shown by the blue and red lines. From
the illustration, opioid alter brain perception of pain through preferential
targeting of the limbic region highlighted in grey. Cognitive control of pain
involving the prefrontal cortex is achieved by opioid activation of
limbic-brainstem inhibition. Figure 2
illustrates the selection process. Figure 2: The selection process. From
the literature, review, opioids are found to modulate several physiological
functions. The degree of impacts is affected by the distribution of opioids
receptors in the brain. The regions documented to show most responses were the
rostral, medial and inferior frontal gyri, cortex, cingulate cortex, and the
precuneus. The prefrontal cortex, insula, amygdala and the cingulate cortex are
parts involved in pain management and are involved in opioid circulation. The
presynaptic effect of opioids in neurotransmitter release is the primary mode
of action of opioids. The mu-opiate receptor is responsible for most of the
observed actions. Opioids trigger a wide range of effect since it interacts
with multiple pathways (Pasternak & Pan, 2013). When opioids infiltrate
into the locus cereleus the user experience slow respiration, low blood
pressure, constipation, and decreased alertness. After long-term use, opioids
can alter the neurological processes, and the brain requires more opioid to
achieve the same reactions, this leads to addiction. Withdrawal is coupled with an extensive
firing by neurons. Thus, rather than constipation and slowed respiration, the
brain begins to trigger elevated blood pressure, and diarrhea (Müller-Lissner
et al., 2016). Instead of happiness, the amygdala triggers feelings of anxiety
and dysphoria. The negative reaction feeds the prefrontal cortex further
promotes the desire opioids. As noted in the research, opioids can cause
profound mood changes, analgesic, tolerance, dependency, and hedonics effects.
Thus, patients taking opioids have to be under strict monitoring, with their
dose and intake always within the recommended levels and period. Most
research on the mechanism of action is based on neurological effects. Cortical
and subcortical brains are directly altered by opioids thus mediating emotions,
impulses, rewards, and motivation. Studies on opiate addicts show alteration of
the white and grey matter morphometric and functional properties. Opioids are
one of the most explored drugs yet, researchers continue to be divided on its
mechanism of action. For decades, it was assumed that opiates have one mode of
action the studies have been refuted by subsequent research that pointed out
differences in mechanisms of action. The involvement of endogenous peptides
brought a new understanding of how opioids interact with the brain discussed in
this paper.Neuro Effects of Opioids on the Human Brain
Abstract
Full-Text
Introduction
Material and
Methods
Results
Discussion
Conclusion
References