Research Article :
Nganso Ditchou Yves Oscar,
Amang A Ngnoung GA, Soh Desire, Simo Nemg Fredy Brice and Nyasse Barthelemy This paper aimed at studying the antioxidant
efficacy of the methanolic leaf extract of Clerodendrum
splendens, a plant of the Lamiaceae family. Phytochemical tests carried out
on extracts of Clerodendrum splendens
leaves have been able to detect the presence of secondary metabolites such as
Flavonoids, Tannins, Saponins and Terpenoids. The results of the antioxidant
activity have shown that CSF2, CSF3 fractions and CSB, CSG fractions similarly
inhibited hepatic lipids but significantly less than vitamin C. Compared to all
fractions, the CSB fraction shows the best inhibitor on the peroxidation of
hepatic lipids because at 150 μg/mL, there is a maximum activity (2.5 μg/mL of
protein). However, it is found that CSF3, CSF2 and CSG have higher IC50 values
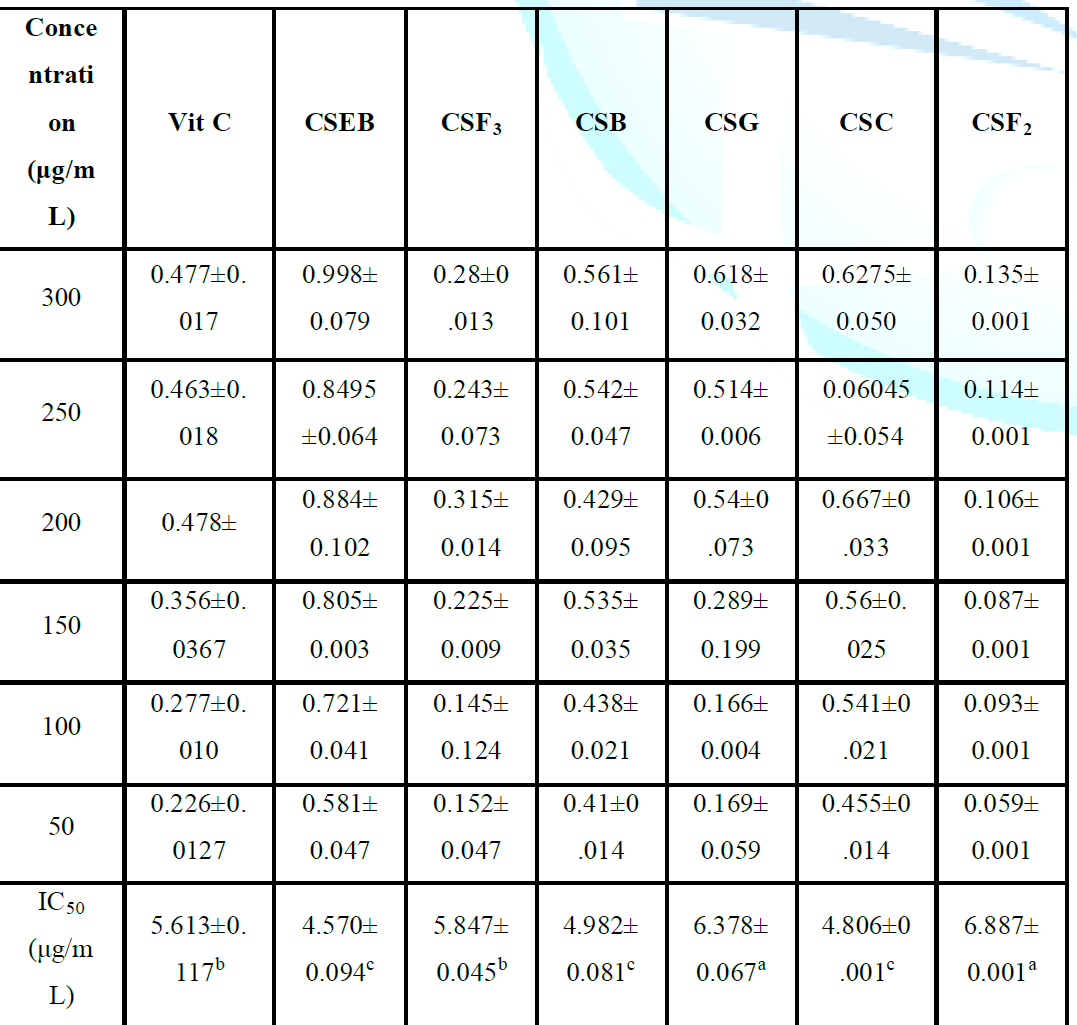
than vitamin C (5.613 ± 0.117) while CSEB, CSB and CSC fractions showed lower
IC50 values than vitamin C, which is used as the reference reducing compound.
The lower the IC50 value compared to vitamin C, the greater the antioxidant
capacity of the plant extract. The results of this study suggest that Clerodendrum splendens represents an
untapped source of compounds with potential antioxidant activity that could be
explored in the development of new therapeutic natural products. Since
ancient times, plants play a very important role in the daily life of men
because they are used as firewood, raw materials in real estate, decoration and
in the treatment of diseases. The use of extracts from different parts of
plants in the preparation of therapeutic potions is a more cultural than social
attribute (Falkenberg et al., 2002).
Today, these are a hive of drugs because they are fully integrated into the
morals of Africa and intervene in traditional pharmacopoeia in the fight
against many diseases such as cancer, malaria, dysentery, yellow fever, ulcers,
gonorrhea (Berhaut, 1975). If medicinal plants are widely used in African
regions and particularly in Cameroon by traditional healers to solve public
health problems, their use requires the expertise of researchers to study the properties
of these plants, to assess the active
dosage and their toxicity
which is often unknown. In
recent years, the natural antioxidants found in medicinal plants are the
subject of several scientific researches as a result of their ability to
prevent the oxidative
stress which is the root cause of several cardiovascular, neurodegenerative
and certain types of cancer. Among the medicinal plants known for their
antioxidant activity, the genus Clerodendrum
is one of them. Interest in the role of antioxidants in human health has
prompted research in the fields of food science and medicinal herbs to evaluate
the role of herbs as antioxidants. Clerodendrun
splendens,
family Lamiaceae
is a shrub about 3.7m tall. It has dark green leaves that are simple and
opposite (Oppong, 2014). The leaves of Clerodendrum
splendens are used in traditional medicine by indigenous people to treat
shingles, spleen in children, asthma, rheumatism, ulcers and malaria (Nnanga et al., 2016). In vivo and in vitro
studies conducted by many researchers have shown that Clerodendrum splendens has antioxidant properties (Okwu and
Iroabuchi 2009 Amal, 2014). The concentration dependent activity of the
methanolic leaf extract of Clerodendrum
splendens annulled the oxidative stress resulting from aggressions. These
results can be used as a prerequisite for screening of plants for bioactive
compounds for medicinal purposes. Antioxidants are compounds that stop molecular
oxidation and play a vital role in protecting the bodys defense mechanism
against free radicals and reactive oxygen species, which are generated
continuously in the body, because of both normal metabolism and some diseases.
(Gate, 1999 Gutteridge and Halliwell 2000) Vitamin C (ascorbic acid), its
antioxidant properties are attributed to its ability to be reduced to ascorbyl
radical after the loss of an electron or a proton. This radical can easily
oxidize by capturing the superoxide anion and some radical species
(perhydroxyls and peroxyls) (Valko et al.,
2006 Antwerpen, 2006). These exogenous antioxidants are also known as natural
antioxidants that exist in food and medicinal plants. Empirical use of
natural antioxidants is a very old practice for food preservation. The search
for new natural antioxidants has been gaining in popularity in recent years, as
the synthetic antioxidants currently used, such as Butylhydroxytoluene (BHT)
and Butylhydroxyanisole (BHA), are not devoid of toxicity (Barlow, 1990).
Phytochemical studies carried out on Clerodendrum
splendens have shown the presence of secondary metabolites including Carbohydrates,
Steroids, Terpenes and Flavonoids (Rohitash et
al., 2012). Thus, the aim of this study was to evaluate the antioxidant
activity of the methanolic leaf extracts of Clerodendrum
splendens (Lamiaceae). After
drying, the leaves of Clerodendrum
splendens were crushed using a grinding machine. Maceration of the powder
was done in methanol in a tightly closed 20 L container. A MARQLUTAN GM-300P
electronic scale was used to weigh the raw extract and the different masses of
the fractions while separation of the extract from the solvent was carried out
using a Büchi brand Heidolph WB 200 rotary evaporator. The
leaves of Clerodendrum splendens were
harvested by Mrs. NDZANA Marie and LEKINI Gisele at Mount Mbankolo in Yaounde
in the Centre Region of Cameroon in September 2016. This plant was identified
in comparison with the sample of Clerodendrum
splendens (G Don) A. Koufani 2009 collector of Clerodendrum splendens (G. Don) of the specimen with a sample from
the National Herbarium of Cameroon under the number 41512/HNC. The
leaves of Clerodendrum splendens were
dried, crushed and a powder of 2836.76 g was obtained. This powder underwent
triple extraction by maceration with pure methanol for 72 hours. The filtrate obtained
was evaporated to dryness using a rotary evaporator under reduced pressure and
188.92 g of crude extract was obtained. 100.08 g of this crude extract was cold
fixed on 90 g of silica gel (SiO2) (0.063-0.200 mm) and the Buchner
was filled with 101.02 g of silica as a stationary phase to undergo flash
chromatography. Elution of this extract was done with solvents and gradient
solvent systems of increasing polarity such as: hexane, hexane/ethyl acetate,
ethyl acetate, ethyl acetate/methanol. We collected 120 fractions of about 400
mL each. The 120 fractions were regrouped into 8 major fractions (A, B, C, D,
E, F, G and H) based on TLC analyses. From these fractions, we had the
following different fractions CSB, CSEB, CSG, CSEB, CSC, CSF2, CSF3. This study
took place at the laboratory of the University of Maroua in Cameroon. Preliminary
phytochemical analysis The
phytochemical creening of methanolic extracts of Clerodendrum splendens was performed according to the standard
procedure by Harbone (Harbone, 1998). All extracts of Clerodendrum splendens prepared were subjected to a preliminary
phytochemical screening for the presence of phenolic compounds, glycosides, anthraquinones,
terpenoids, flavonoids,
tannins, carbohydrate, lignans and saponins. Antioxidant
activities Catalase
activities Catalase
activity procedure:
In the spectrophotometric vats (white and tests), 200 μL of H2O2
(9 %) were introduced 1150 microliters and 1135 μL of phosphate buffer (0.1 M
pH 7.2) were respectively introduced into the white test tanks. 15.5
microliters of DMSO 0.025 % (white tank), Vitamin C or CSB, CSEB, CSG, CSEB,
CSC, CSF2 (for final concentrations of 0, 50, 100, 150, 200 and 250 μg/mL) were
added in the vats. Two hundred microliters of hepatic PMS were then added to
the test tanks and after homogenization, the ODs were read at 240 nm at 30, 60
and 90 s. The enzymatic activity of catalase, expressed in IU/mg of testicular
PMS protein, was calculated. Principle:
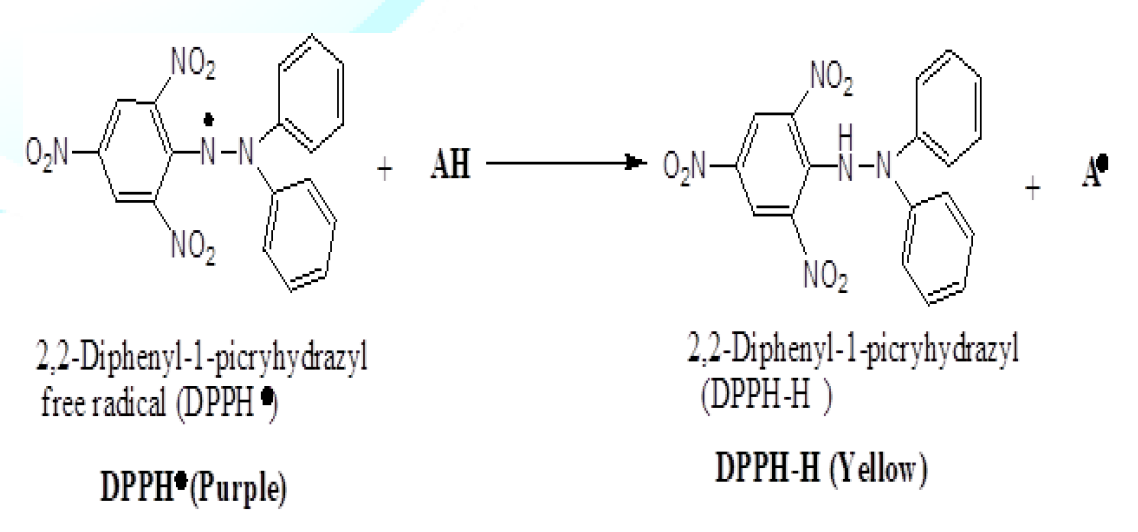
The
DPPH° radical is trapped directly by an antioxidant (AH) which gives it a
hydrogen atom and reduces it to DPPH-H. This results in a color change in the
DPPH methanolic solution that gradually changes from purple to yellow. This
color change is measured at λ=517 nm (Gokhan et al., 2010). Figure 1: DPPH
Free radical conversion to DPPH-H by antioxidant compounds. Procedure:
50
μL of extract was added to each test tube containing 3.1 mL of the methanolic
solution of DPPH 40 μg/mL. In negative control tubes, the extract was replaced
with 50 μL of DMSO. The mixtures were well homogenized and incubated in the
dark for 30 minutes at room temperature. The absorbances read at λ=517 nm was
used to calculate the trapping percentages, the trapping concentrations fifty
(CP50), the effective concentrations fifty (EC50) and finally the anti-free
radical powers (PA) according to the formulas: Trapping
activity of the OH° radical Principle:
In
the presence of FeSO4 and H2O2, the hydroxyl
radicals (OH°) are formed. The latter, coupled with sodium salicylate, form a
violet complex which absorbs at λ=562 nm. The intensity of the coloration is
inversely proportional to the amount of free radical in the medium (Zhenwei et al., 2009). Procedure:
In
each test tube, 50 μL of polyphenol extract, 0.7 mL of FeSO4 (3 mM),
1 mL of H2O2 (1 mM), 1 mL of distilled water and 0.4 mL
of sodium salicylate were added (10 mM). In the negative control tubes, the
extract was replaced with 50 μL of DMSO while the white contained distilled
water instead of sodium salicylate. The mixtures were incubated at 37°C for 1
hour and the absorbances were read at λ=562 nm against the white. The different
percentages of entrapments were calculated using formula (1) (Zhenwei et al., 2009). Ferric
Ion Reduction Capacity (FRAP) Procedure of Ferric
Ion Reduction Capacity (FRAP) In
test tubes (blank, assays), 200 μL of freshly prepared FRAP reagent (potassium
ferricyanide 1 %) and 500 μL of phosphate buffer (white test tube), vitamin C
or CSB, CSEB, CSG, CSC CSF2 (for final concentrations of 0, 50, 100, 150, 200
and 250 μg/mL) are introduced. In parallel, 200 μL of FRAP reagent and 10 μL of
a ferrous sulphate solution (to obtain the final concentrations of 0, 50, 100,
150, 200 and 250 μg/mL) are introduced into the standard test tubes. All the
tubes are incubated for 20 min at 50°C, 500 μL of 10 % TCA added and the ODs
read at λ=593 nm. The Fe2+ TPZ concentrations characterizing the
reducing activity of the products are determined from the OD calibration curve
as a function of the ferrous sulphate concentration. Reducing
activities Potassium ferricyanide reduction test Principle:
This
test is based on the reduction of potassium ferricyanide K3[Fe(CN)6]
to potassium ferrocyanide K4[Fe(CN)6] by an antioxidant.
This reduction results in the change of the yellow color of the solution to
green in the presence of ferric chloride (FeCl3) and the absorbance
of the solution is read at λ=700 nm (John et
al., 2010). Procedure:
In
each test tube were successively introduced 50 µL of extract, 1 mL of phosphate
buffer (0.2 mM, pH 6.6) and 1 mL of potassium ferricyanide (1% w/v). The
mixtures were incubated (50°C, 20 minutes). After incubation, 1 mL of
trichloroacetic acid (TCA) 10 % w/v was added and the mixtures centrifuged
(3000 rpm, 10 minutes). To 1 mL of aliquot of each mixture, 1 mL of distilled
water and 0.2 mL of ferric chloride (0.1 % w/v) were added and the absorbances
were read at λ=700 nm (John et al.,
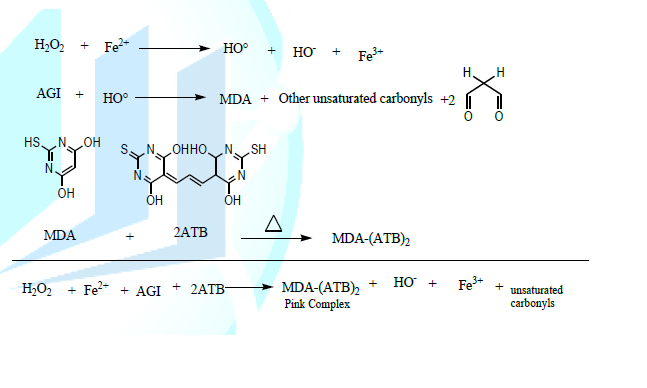
2010). Initiation and
inhibition of lipid peroxidation in rat liver homogenate Principle: Coupled with Fe2+,
H2O2 releases the hydroxyl radical (HO°) which attacks
the ethylenic bonds of unsaturated fatty acids (AGI) to oxidize them. Reactive
acid substances are formed with Thiobarbituric acid (ATB), including
malondialdehyde (MDA), which reacts in acid medium and when heated with two
molecules of ATB to form a pink complex that absorbs at λ=532 nm according to
the equation below (Xiao-Yu et al.,
1989). Procedure
for the inhibition of lipid peroxidation: 800 μL, 1000 μL and 900 μL of
Phosphate buffer (50 mM, pH 7.4) were introduced into test tubes: blank,
control and test, respectively. One hundred microliters of hepatic homogenate
(volume for a final protein content of 2.3 mg/mL in the tube) are added to the
test tubes followed by 100 μL of Vitamin C, or CSB, CSEB, CSG, CSC, CSF2 (for
final concentrations of 0, 50, 100, 150, 200 and 250 μg/mL). Lipid peroxidation
is initiated by adding 100 μL 1M Fe(SO4)2 to all tubes.
After a 15 min incubation period at 37°C, the lipid peroxidation is stopped by
adding 1 mL of 20 % TCA and then revealing the malonic aldehydes by adding 1 mL
of 0.67 % TBA to the tubes. One hundred microliters of hepatic homogenate is
then added to the control tube. The tubes are heated at 90 °C for 10 min,
cooled and centrifuged at 3000 rpm for 15 min at 4 °C. The supernatants are
removed and the ODs are read at 530 nm. The percent inhibition of lipid
peroxidation (% I) is calculated using the following formula (Xiao-Yu et al., 1989). Results and
discussion Results The
leaves of Clerodendrum splendens
harvested at Mount Mbankolo in Yaounde capital of Cameroon in September 2016,
were cut, dried, crushed and extracted with methanol
at room temperature. These different extractions made it possible to split into
several extracts that the following codes were assigned: CSB, CSEB, CSG, CSEB,
CSC, CSF2, CSF3. CSB: Fraction B CSC: Fraction C CSG: Fraction G CSF2 et
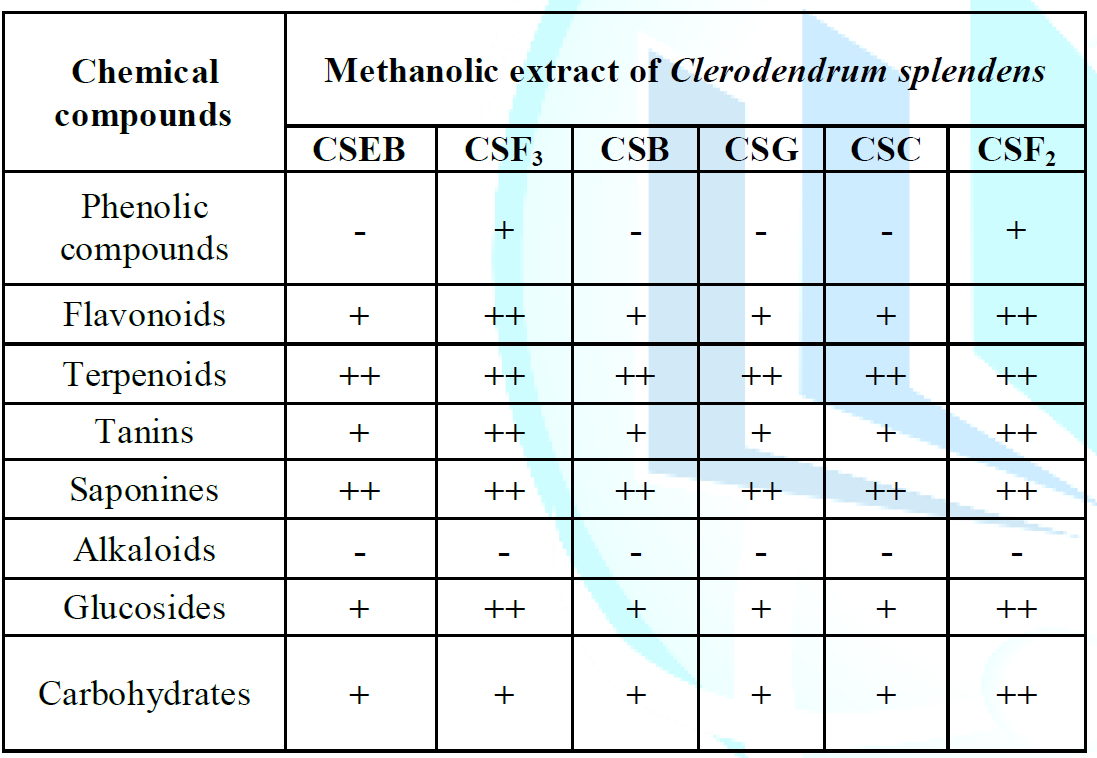
CSF3: Fraction F. Qualitative
phytochemical screening of Clerodendrum splendens
crude extracts showed the results presented in Table 1. From these results, a number of classes of metabolites
were identified among all extracts of Clerodendrum
splendens, among which Sterols, Alkaloids, Flavonoids, Sugar glycosides,
Saponins, Quinones, Polyphenols, Triterpenes and Saponins were tested on
extracts of Clerodendrum splendens. From
the table below, the results show that Clerodendrum
splendens leaves contain in abundance secondary metabolites such as
Terpenoids, Saponins, carbohydrates and glucosides. They contain traces of
Flavonoids and Phenolic compounds and alkaloids are completely absent. These
results agree with the conclusions of many authors who identified the same
classes of chemicals in extracts of roots, bark stems and leaves of Clerodendrum splendens (Burkill, 1988). Table 1:
Phytochemical Screening of Methanolic extracts of Clerodendrum splendens. Antioxidant
activities Catalase
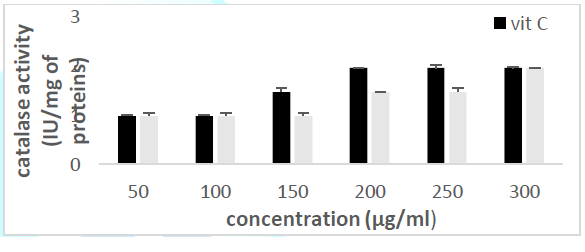
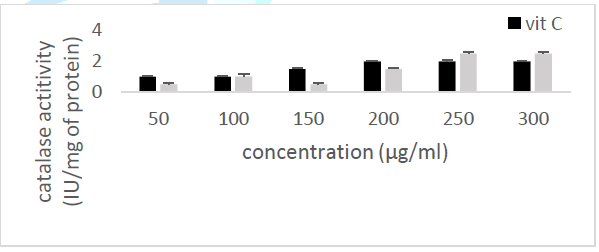
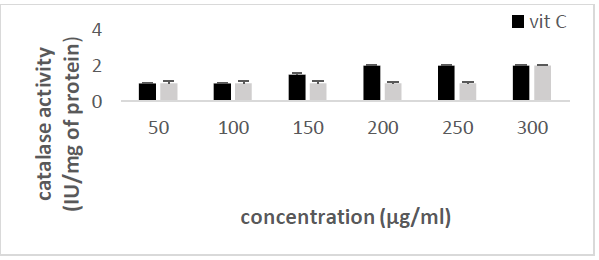
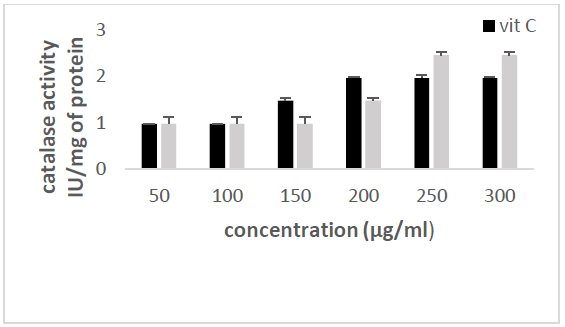
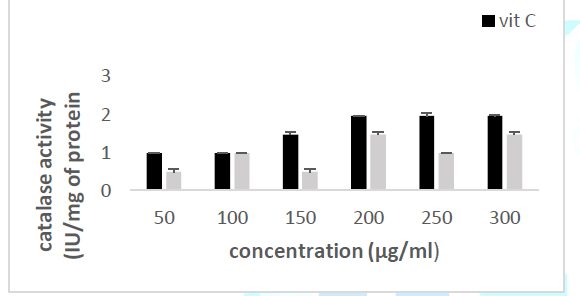
activity Principle: Catalase is an
enzyme in the antioxidant cell system that catalyzes the decomposition of hydrogen
peroxide (H2O2) to give water and oxygen. The higher
the catalase activity as compared to vitamin C (the reference antioxidant
compound), the more beneficial the plant is to the human system. At 240 nm, the
absorbance of the non-degraded hydrogen peroxide is directly proportional to
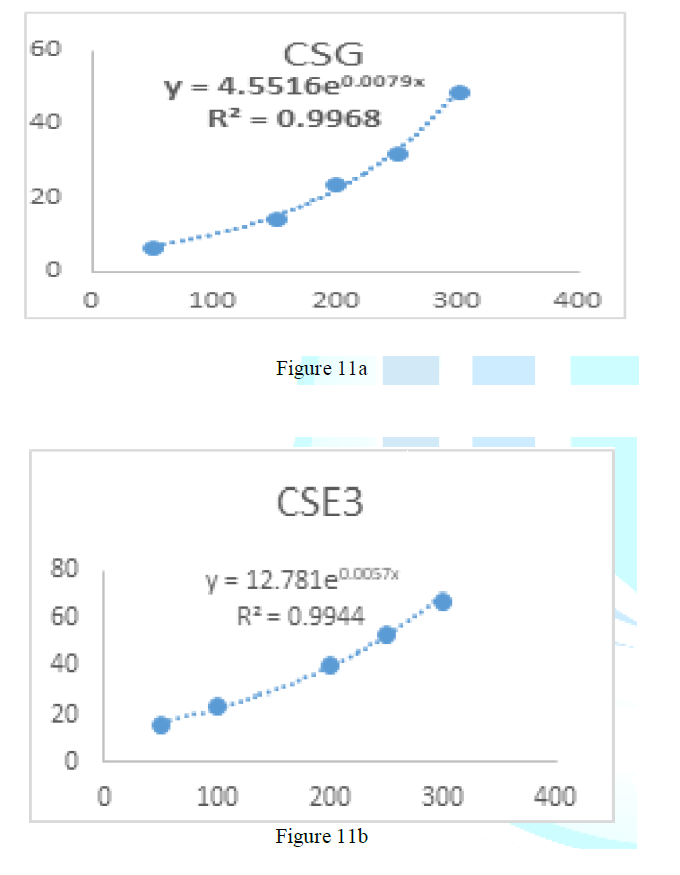
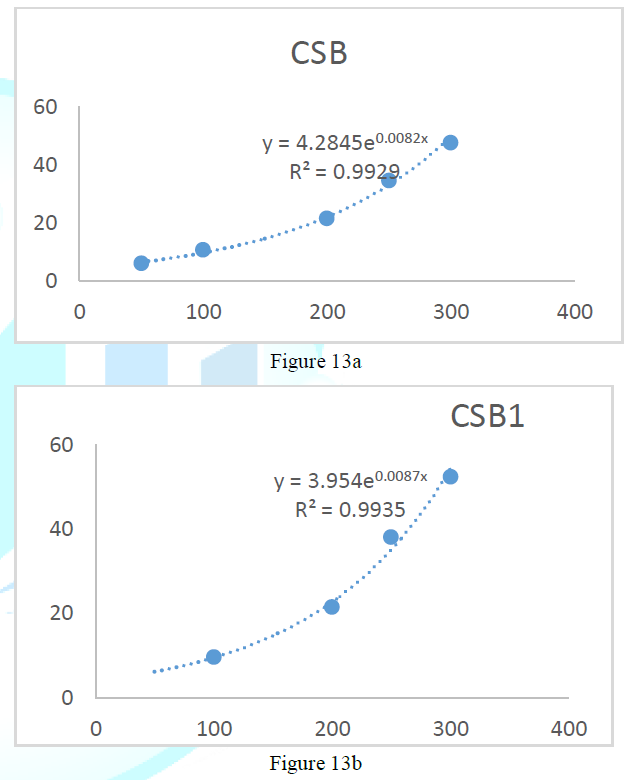
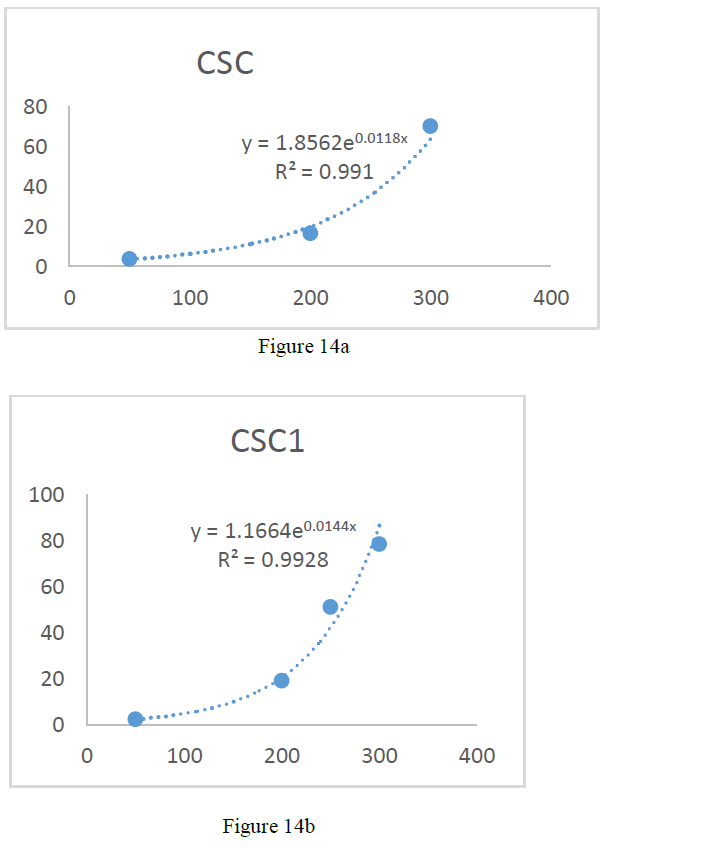
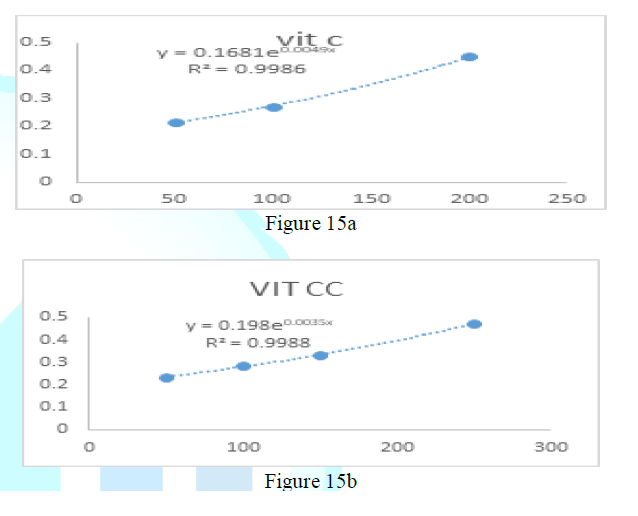
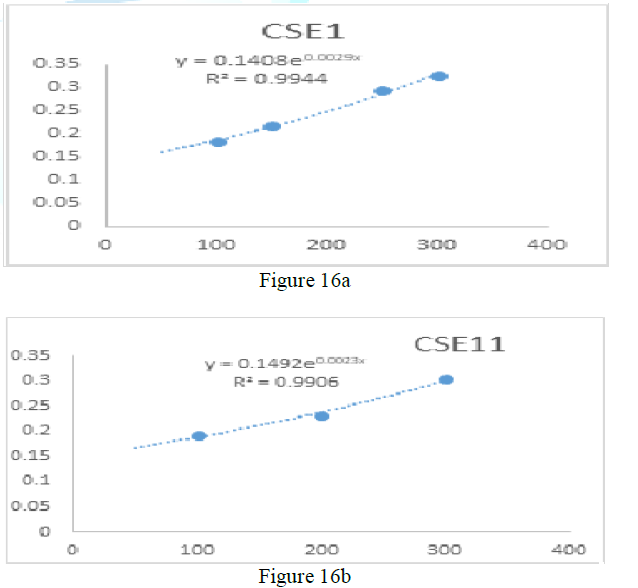
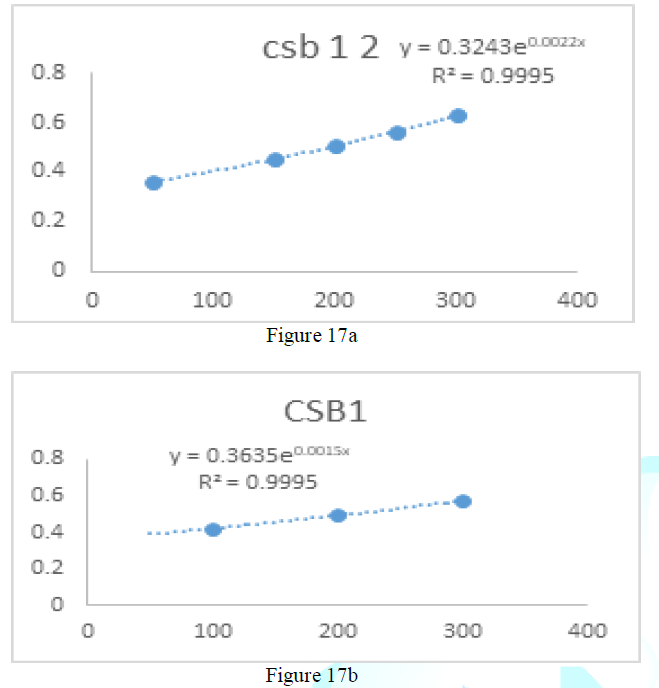
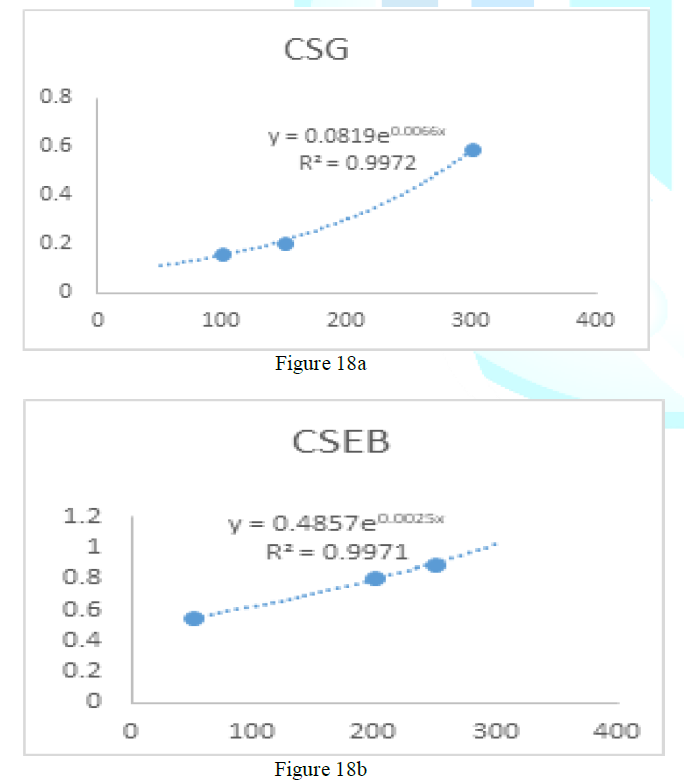
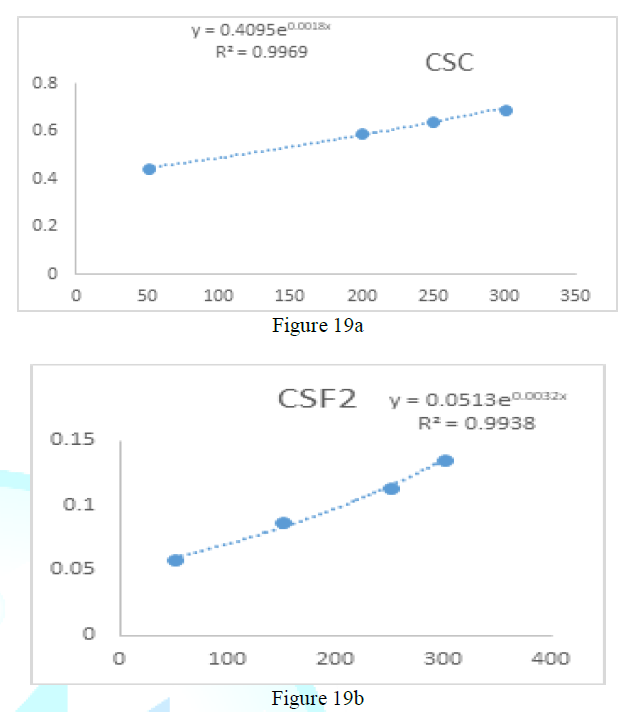
the catalase concentration of the sample (Misra and Fridovich 1972). Figure 3: Effect
of CSB crude extract. Figure 4: Effect
of CSE3 crude extract. Figure 5: Effect
of CSG crude extract. Figure 6: Effect
of CSEB crude extract. Figure 8: Effect
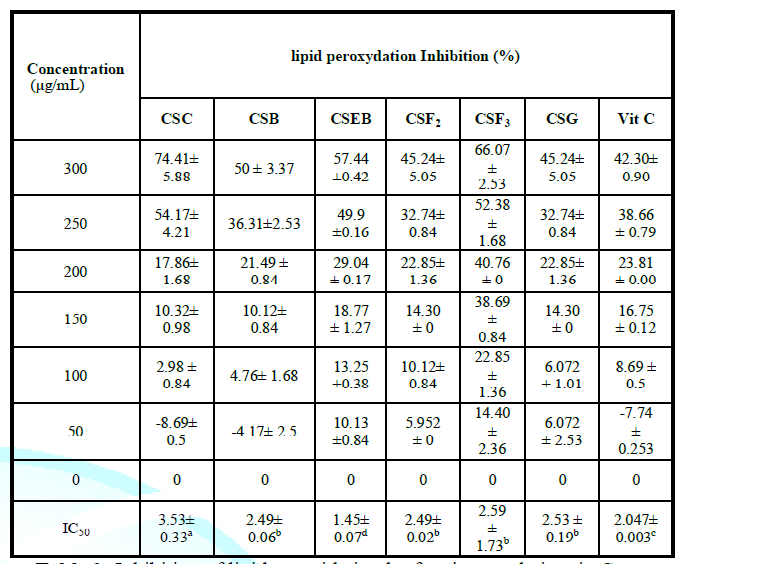
of CSF2 crude extract. Inhibition of
the peroxidation of hepatic lipids The different
fractions inhibit the peroxidation
of hepatic lipids (Table 2).
Compared with each other, CSF2, CSF3 and CSB, CSG fractions similarly inhibit
hepatic lipids but significantly less than Vitamin C and CSB. Compared to all
the fractions, the CSB fraction shows the best inhibitor on the peroxidation of
hepatic lipids because at 150 μg/mL, there is a maximum activity (2.5 μg/mL of
protein). From this table, it appears that extracts of Clerodendrum splendens significantly inhibited lipid peroxidation
in a concentration dependent manner. This allowed us to determine the IC50 of
the different extracts. The CSB, CSG, CSC, CSF2 and CSF3 extracts had higher
IC50 values than vitamin C (2.047±0.003) while the CSEB fraction had a lower
IC50 value than vitamin C (1.45±0.07). Inhibition of lipid peroxidation was
done according to method by Pilipenko (Pilipenko, 1990).The supernatants are
removed and the ODs are read at 530 nm. The percent inhibition of lipid
peroxidation (% I) is calculated using the formula (Fig. 9) Table 2:
Inhibition of lipid peroxidation by fractions and vitamin C. Evaluation of
ferric ion reduction capacity (FRAP) Principle: This method
evaluates the ability of an antioxidant to transfer electrons to Fe3+
ions. These ions are in solution in the form of Fe3+/2,4,6-tripyridyl-S-triazyn
(TPZ) complex and their reduction gives the blue complex Fe2+/2,4,6-tripyridyl-S-triazyne
which absorbs at 593 nm (Benzie and Strain 1972). The intensity of blue color
depends on the reducing power of the molecule tested. All the extracts have a
reducing effect on Fe2+ (Table 3). The CSG and CSF2 fraction have a
comparative reduction effect on Fe2+ ions, a lesser effect compared
to Vitamin C and the CSEB fraction. The fractions CSEB, CSB and CSC have better
effects on the reduction of ferric ions. Of these fractions, the CSEB fraction
is the most reductive of ferric ions. Table 3:
reduction ferric ions by the fractions extracts of Clerodendrum Splendens and
vitamin C. From
the table below, it appears that all extracts of Clerodendrum splendens showed a reducing power which is directly
proportional to the concentration of extract compared to that of vitamin C
which allowed determines the IC50 of the various extracts. Discussion This
work has successfully determined the antioxidant capacity of the methanolic
extract of Clerodendron splendens.
Catalase
is an enzyme that is responsible for the degradation of hydrogen peroxide in
water and molecular oxygen. This enzyme is involved in the bodys defense
mechanism against infectious agents. Hence, the more a plant extract increases
the activity of this enzyme is as high as compared to that of the reference
compound (Vit C), the more this extract will be beneficial for the body. As
part of this work, we were able to determine the IC50 of plant extracts. This
result makes it possible to say that the activation of the catalase activity
does not depend on the concentration of the extract. The
ability of extracts to protect biomolecules
against oxidation was assessed by measuring their ability to inhibit lipid
oxidation. Indeed, these lipids are oxidized in a reaction cascade whose
increase in concentration is a sign of significant membrane lipid peroxidation.
Some extracts showed lower IC50 values than vitamin
C, which shows that these extracts may consist of polyphenols and Flavonoids
which are found in literature review for their great antioxidant power. These
results corroborate those of Xiao-Yu (Xiao-Yu et al., 2009) which in fact obtained a strong inhibition
of the lipid peroxidation of the rat liver homogenate with the ethanolic
extract of Clerodendrum splendens.
Since lipid peroxidation has been induced in the rat liver homogenate by Fe2+
and H2O2 ions, we can think that its inhibition by plant
extracts could be attributed to their ability to either chelate iron or to trap
the radical. OH° from the Fenton reaction. Conclusion Fractions
of the methanolic leaf extract of Clerodendrum
splendens were found to have pharmacological secondary metabolites
Saponins, Terpenoids and Tannins in abundant quantities, while Alkaloids do not
exist. This indeed suggests that the methanolic leaf extract of Clerodendrum splendens is a relevant and
potential antioxidant. Thus, describing the leaf extract of Clerodendrum splendens as possessing
activities of attenuations of oxidative stress and hepatopathy. Stress related
to stress-induced cold stress was confirmed by deleterious effects on the
physiological and immune systems against age-related stressors and macular
degeneration. However, the concentration-dependent activity of the methanolic
leaf extract of Clerodendrum splendens
annulled the oxidative stress resulting from these aggressions. These results
can be used as a prerequisite for Clerodendrum
splendens screening of plants for bioactive compounds for medicinal
purposes. Acknowledgement The
authors thank the Department of Chemistry of the Faculty of Science of the
University of Maroua and Mr. Simo Nemg Fredy Brice, of the Laboratory of
Pharmacology and Toxicology, Department of Biochemistry of the Faculty of
Sciences of the University of Yaounde1 for antioxidant tests. ReferencesPhytochemistry and Antioxidant Activities of the Methanolic Leaf Extract of Clerodendrum splendens (Lamiaceae)
Abstract
Full-Text
Introduction
Material and Methods
Apparatus and
equipment
Plant material
Maceration of
the Plant Material
Trapping activity of the radical DPPH° (1, 1-diphenyl-2-picrylhydrazyl)