Research Article :
One of the most important goals
of root
canal treatment is to eliminate bacteria from the
infected root canal systems through instrumentation and use of disinfecting
agents [1,2]. Biofilm can be defined as a sessile multicellular microbial community
characterized by cells that are firmly attached to a surface and enmeshed in a
self-produced matrix of Extracellular
Polymeric Substance (EPS) [3,4]. Elimination from
anatomical complexity of the root canal system is a challenging procedure. Thus; the use of a potent antibiofilm strategy has become mandatory to be able to resist endodontic infection [5,6]. Sodium hypochlorite
in concentrations from 0.5%-6% is the most commonly recommended root canal irrigant. Aqueous solution of sodium hypochlorite is a dynamic balance of sodium
hydroxide and hypochlorous acid, which on interaction with microorganisms and
organic tissue causes chloramination, amino acid neutralization, and
saponification reactions leading to strong antibacterial and tissue-dissolving
effects [7]. Calcium hydroxide is the most commonly used intracanal medication during root canal procedures [8]. Its antibacterial property is generally
related to the release of hydroxyl ions, which produces the lethal effects on
bacterial cells including protein denaturation and damage to the bacterial cytoplasmic
membranes and DNA [9]. However, the antimicrobial activity of sodium
hypochlorite and calcium hydroxide can be inactivated by dentin, exudate from
the periapical area, and microbial biomass [10]. In addition, both sodium
hypochlorite and calcium hydroxide do not always eliminate E. faecalis biofilms from the root canal system. Recently, Photodynamic Therapy (PDT) as an antibiofilm strategy is based on the use of a nontoxic dye
(photosensitizer agent), that when activated by using a low energy light lead
to production of free radicals such as singlet oxygen [11]. Singlet oxygen
generated is highly reactive and is known to target various bacterial sites
such as cell wall, nucleic acid, lipids membrane and proteins membrane, which
promote bacterial cell death. Therefore, it has potentiality to enhance the
disinfection efficacy of the conventional chemo-mechanical preparation [12]. Nanoparticles
are insoluble particles that are ranging from 1 to 100 nm in size which
combines biology principles with physical and chemical procedures to generate
nano-sized particles with specific functions [13]. Different nanoparticles have
been introduced as an irrigant or medicament to control the bacterial biofilm
in root canal system. Pomegranates (Punica granatum
L.) which have a long history of antibacterial use dating back to biblical
times [14]. Egyptians used
pomegranates to treat a number of different infections. It was utilized as a
traditional remedy for thousands of years under the Ayurvedic system of
medicine, with extracts from the rind of the fruit and bark of the tree being
effective against diarrhea and dysentery. The Pomegranates in nanosized form
have been applied in many health care fields because of their broad-spectrum
bactericidal and virucidal properties [15]. So, the aim of this study was to
evaluate the antibiofilm efficacy of nanoherbal medicament and photodynamic
therapy using Confocal Laser Scanning Microscope. A total of one
hundred non-carious recently extracted human single rooted teeth with fully
formed root apices were collected from patients with ages ranging between 20
and 45 years old. Also to confirm the presence of type I root canal morphology
in each tooth according to Vertuccis classification. The teeth that had caries, deep cracks, attritions,
fractures or restorations would be excluded from the study. The dentin
section blocks were prepared and standardized to be (4×4×1 mm) (Length × Width
× thickness) according to Haapasalo and Orstavik D. technique through the
following steps: Demarcations were done at Cementoenamel Junction (CEJ) and at
the last 3 mm of the roots. The crowns of demarcated teeth were cut off at the
level of CEJ by using a diamond disc mounted in a straight hand piece under
water coolant [16]. Root canal patency for each sample was done using a size
#10 and #15 K file. Instrumentation was done using Revo-S rotary NiTi file system according to the manufacturers instructions. Rotary files
were mounted in a torque limited control motor at a torque 1.8 Ncm and speed
400 rpm as recommended by the manufacture starting from SC1 (25 taper 0.06) to
AS40 (40 taper 0.06). Irrigation was
done using side vented needle gauge 30 mounted on 3 ml plastic syringe of 5.25%
Sodium hypochlorite (NaOCl) between each instrumentation during the cleaning
and shaping procedure. The apical 3 mm of the roots were sectioned off and the
roots were longitudinally sectioned in bucco-lingual direction along the
midsagittal plane into two semicylindrical halves and the cementum was removed
from the root surface using Isomete saw at 1000 rpm under water cooling. Each
samples of the roots were shaped and refined to be (4 × 4 × 1 mm) (Length ×
Width × thickness) respectively using Isomete saw at 1000 rpm under water coolant. Each dentin
section block was placed in 1.5 mL Eppendorf tube filled with Brain Heart Infusion (BHI) broth and sealed within sterilization pouches then underwent sterilization
using autoclave at 12 °C for 20 minutes. After completion of
sterilization, the Eppendorf tubes opened in a sterile air laminar flow cabinet
and a sterile paper points were inserted into the Eppendorf tubes for 1 minute
until it completely saturate and absorbed the broth media, then the paper point
was transferred and spread on brain heart infusion agar plate The plate was
incubated for 24 hours at 37°c and 100% humidity for bacterial
count. After 24 hours the plate was inspected and checked for no inhibition
zones around the incubated paper point indicating a sterility of the samples
before inoculating the multispecies microorganisms. Cultivation of Standard strains: E. faecalis American Type Culture Collection (ATCC 29212) and Staphylococcus epidermidis (Staph.
epidermidis) American Type Culture Collection (ATCC 12228) were done and each
Eppendorf tube contain sterile dentin section block was opened in a sterile air
laminar flow cabinet. Eppendorf tubes were filled with 0.5 ml of E. faecalis suspension plus 0.5 ml of Staph
epidermidis suspension using a sterile micropipette tips for each organism.
Eppendorf tubes were closed tightly and shacked well then incubated for 21 days
at 37°C in incubator. After incubation
period, the samples were examined for confirmation of multispecies biofilm
formation on dentin section blocks. Three randomly samples were selected after
finishing of sterilization and before bacterial inoculation. Also, others three
randomly samples were selected after 21 day after incubation of dentin section
blocks with multispecies microorganisms. These samples were inspected to
confirm the presence/absence of multispecies bacterial biofilm formation using Scanning Electron Microscope (SEM) as shown in (Figure
1a and Figure 1b). Figure
1a: Image showing dentin section block
immediately after sterilization. Figure
1b: Image showing dentin section block
after 3 weeks of biofilm inoculation. Preparation
of nano pomegranate extract: The herbal extracts are turned
into nano herbals by nano emulsion O/W (oil on water) which was done using
two-step procedure, Initially coarse emulsion was prepared by mixing the
paraffin oil (1 ml)+(1 gram extract) and surfactant Tween 80 (2 ml) followed by
addition of distilled water (7 ml) in the ratio 1:2:7 using magnetic stirrer at
600 rpm for 10 min at 25°c. Then, prepared coarse emulsion was
further sonicated using sonicator with a maximum power output of 750 W under
ice bath to avoid rising in temperature. The sonication process was carried out
for 10 minutes. Characterization
of transformation to nanoherbals: Nanoemulsion
was characterized by Transmission
Electron Microscopy (TEM) at magnification 20000 and
it showed nanoemulsion spherical in shape with average size of 50 nanometer (Figure 2). Rose
Bengal dye (Photosensitizer agent): Stock
solution of Rose
Bengal dye (Sigma-Aldrich, St Louis, MO. USA) was
prepared by dissolving 20 mg RB powder in 20 ml deionized water to obtain a
final concentration 10 μM/mL of RB dye after vortexed for 3 min at 37°C.
The stock solution of Rose Bengal dye was stored at 4°C when not needed. Characterization
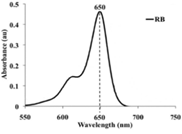
of Rose Bengal (RB) dye (Spectrophotometric analysis of RB dye solution):
The absorption spectra of diluted solution of the prepared RB dye was recorded
within 550 to 750 nm using double beam UV-Visible Spectrophotometer**
and the maximum absorption peak was recorded at 650 nm (Figure 3). Figure
3: A diagram showing UV-absorption spectrum of RB
showed maximum absorption peak at 650 nm. Group
(1)-Medicated with nano punica granatum
herbal extract medicament: Each sample was medicated with 1
ml of nano punica granatum herbal
extract medicament within the Eppendorf tube till its fully covered and
incubated in the incubator at 37°C for 7 days at humidity 100%. Group
(2)-Medicated with Calcium hydroxide medicament: Each
sample was medicated with 1 ml of Calcium hydroxide medicament (Urbical
promedica Germany) within the Eppendorf tube till it is fully covered and
incubated in the incubator at 37°C for 7 days at humidity 100%. Group
(3)-Final rinse using Rose Bengal dye (RB) and activated with diode laser
(Photodynamic therapy): Each sample was rinsed with 1 ml
of Rose Bengal dye solution (10 μM) concentration within the Eppendorf tube and
protected from ambient light then left for 15 minutes (prior to irradiation)
for allowing the dye to interact with biofilm. Irradiation was performed using
a diode laser (Lasotronix device) 635 nm for 5 minutes at energy fluence 30
J/cm2. Group
(4)-Final rinse using Sodium Hypochlorite (NaOCl):
Each sample was rinsed with 1 ml of Sodium Hypochlorite (NaOCl) solution 5.25%
concentration within the Eppendorf tube then left for 15 minutes. Group
(5)-Control group: There is no final rinse was used
in this group. Each sample was left within the Eppendorf tube without any
treatment (Negative control group) until time of evaluation. For the analysis of antimicrobial
activity using confocal laser scanning microscopy, the samples were removed
from the nanoherbal gel and irrigant solutions then washed for 1 min by
deionized water to remove the remnants of the nanoherbal
medicaments and irrigant solutions then stained by
(Florocine Diacetate FDA and Propodium Iodid PI) to detect Live/Dead bacteria
in the bacterial biofilm and left for 15 min then the samples were removed from
the stains solutions, placed on slide, covered by cover slide and examined
under confocal microscope then, the data were collected, tabulated, and then
statistically analyzed. The mean and standard deviation
values were calculated for each group. Viable counts of antibacterial activity
were transformed to their log10 values. Data were confirmed to be normally
distributed. All Data were explored for normality using Kolmogorov-Smirnov and
Shapiro-Wilk tests. Data showed parametric (normal) distribution. One way ANOVA
followed by Tukey post-hoc test was used to compare between different groups
for non-related samples in parametric data. Pair-wise sample t-test was used to
compare between dependent samples. The significance level was set at P ≤ 0.05.
Statistical analysis was performed with IBM® SPSS®
Statistics Version 20 for Windows. With regard to the antimicrobial
effectiveness of tested irrigant and medicaments, the highest mean percentage
of dead bacteria was found in nano punica granatum
(Group 1) (88.38 ± 5.45) followed by Rose Bengal dye (RB) (Photodynamic
therapy) (Group 3) (75.73 ± 5.15) followed by sodium hypochlorite 5.25% (Group
4) (59.43 ± 8.14) followed by calcium hydroxide paste medicated (Group 2)
(38.73 ± 5.15). The least mean percentage of dead bacteria was found in Control
group (Group 5) (7.25 ± 1.12). With a statistically significant difference
between groups where (p<0.001) as shown in Table 1 and Figure 4. Analysis
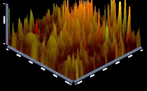
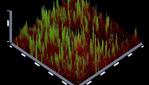
of multispecies biofilm images by CLSM: Figures 5-10 showing 3D reconstruction
of multispecies bacterial biofilm representing live (green) and dead (red)
bacteria for different groups. Figure
5: Group (1) Nano punica
granatum. Figure
6: Group (2) Calcium hydroxide. Figure
7: Group (3) Rose Bengal Dye (RB dye). Figure
8: Group (4) Sodium hypochlorite (NaOCl). Figure
9: Group (5) Control Group. Root canal disinfection is one of
the main clinical challenges in endodontic
therapy due to the physical limitations of
irrigation within a closed system, anatomical root complexities and bacterial
accumulation in the forms of bacterial biofilms [17,18]. Many methods have been
utilized to efficiently disinfect the root canal system including different
irrigant solutions with different activation method [19]. The level of biofilm
maturation is an essential factor for biofilm resistance to antimicrobial
agents [20]. For the biofilm development; the bacterial suspension was changed
every 3 days to avoid the bacterial endotoxins accumulation that might have led
to bacterial death. This allowed the bacterial multiplying without being
dampened by their byproducts [21]. The most potent antibacterial
compounds in pomegranate is ethanol, water, methanol, and acetone extracts of P. granatum that have shown strong
antimicrobial properties against gram-positive and gram negative non-oral
microorganisms also, contribute synergistically as mixtures to bring about the
effects, including anthocyanins (pelargonidin-3-galactose and
cyanidin-3-glucose) and flavonols (quercetin and myricetin) [22]. Although many
intracanal
medicaments were introduced to be used to disinfect
root canal, calcium hydroxide remains the most wildly used as it exerts
antibacterial effects in the root canal system as long as a high pH is
maintained [23]. The Rose Bengal dye was selected as a photosensitizer agent
for Photodynamic therapy due to its antibacterial activity against various gram
positive and gram negative bacteria [24-26]. Also, RB dye is an anionic PS
that has been shown excellent binding with cationic polymers such as chitosan
nanoparticles. The concentration of anionic RB dye that used in the study was
10 μM. At this concentration, the singlet oxygen that produced was enough to
induce significant biological activity against bacterial biofilm and this
concentration also was founded to be below the cytotoxic limit based on the
Shrestha and Kishen study [24]. Confocal Laser Scanning Microscope was used in
this study because of its ability to determine the microbial biofilms at
different levels of the infected root canal. Also, it provides detailed
information about the presence, distribution, viability status, and depth of
penetration of biofilm inside dentinal tubules in accurate 3D reconstructions
images. The viability status of bacterial biofilm (live/dead bacteria) was
determined throughout use of fluorescence-based live/dead staining technique.
This technique were performed with two fluorescent dyes that was allowed two
color discrimination of the population of living cells from the dead cells
population (green/red colored). FDA and PI stains were used to stain live cells
and dead cells, respectively [27]. FDA is a non-fluorescent
permeable dye, when cross the cellular membrane of
living cell convert into fluorescein dye (green in color) by interaction with
intracellular Esterases (produced by metabolic activity of microorganisms). PI
is a fluorescent impermeable dye that cannot cross the cellular membrane of
living cells. It reaches the nucleus by passing throughout disordered areas of
dead cell membranes and staining the nuclei (red in color) by intercalate with
the DNA double helix of the cell. Thus, live bacterial cells are fluorescent
green, whereas dead bacteria with damaged membranes are fluorescent red in
color [28]. In the present study, the control
group (group 5) showed the least mean percentage of dead bacteria when compared
to other groups. The percentage of dead bacteria was found to be 7.25 % which
is the normal for any untreated bacterial population. This result is in
agreements with other researches done in this filed [29,30]. This can be
explained by the fact of the absence of disinfecting agent in the control group
that has lethal effect on the multispecies bacterial biofilm. With regard to
pomegranate nano-herbal (group 1) it showed the highest significant absolute
mean score of dead bacterial cells and these results were coincided with
results reported by shoko T. et al. these findings may be attributed to that
pomegranate extracts contains phenolic, anthocyanin, flavonols and organic
acids. In accordance to our study the antimicrobial properties of P. granatum have been recently noticed
[31,32]. The ethanol, water, methanol and
acetone extracts of P. granatum have
shown strong antimicrobial properties against gram-positive and gram negative
nonoral microorganisms [33]. However A few studies have evaluated the
antibacterial properties of this plant on oral bacteria [34]. The effect of
water extract of P. granatum flower
(petal) on oral microbial pathogens was investigated, which showed its greatest
antimicrobial effect on Streptococcus
sanguinis. Also in accordance to the present study, Gulube and Patel investigated
pomegranate on biofilm formation, the crude extract of pomegranate killed
cariogenic Streptococcus mutans at
high concentrations [35]. At sub-bactericidal concentrations, it reduced
biofilm formation. Irshad R. et al. revealed that
pomegranate nanoparticles are found to have high antibacterial activity along
with biocompatibility [36]. This high antibacterial activity can be referred to
its small size and large surface area. With regard to calcium hydroxide
medicament (group 2) the multispecies biofilms on dentin section blocks were
destroyed after 7 days of treatment, whereas several live bacteria still
remained on the dentin sections. Possibly the high alkalinity of calcium
hydroxide was neutralized by the dentin and biofilm matrix of multispecies
biofilms [37,38] attributing to the reduced antibacterial effect of calcium
hydroxide. With regard to RB dye group
(group 3), the result of dead bacteria could be attributed to the fact of RB
dye is an anionic photosensitizer (negatively charged photosensitizer) that
does not interact electrostatically with the negatively charged bacterial cell
membranes, resulting in the membrane barriers of the bacterial cells limit the
simple diffusion of RB dye into the bacteria cells. This leaded to reduced
penetration of the Rose Bengal dye in to bacterial
biofilm within dentinal
tubules [39]. Also, this result is in
agreements with other researches done in this field [40,41]. With regard to sodium
hypochlorite group (group 4), the result of dead bacteria could be attributed
to the fact of sodium hypochlorite in concentration 5.25% demonstrated
antibiofilm effect against E. faecalis
biofilm. The proportion of live bacteria in the 5.25% sodium hypochlorite group
could be measured by viability staining and CLSM because sodium hypochlorite
destroyed E. faecalis biofilm so
quickly, leaving very little residual biofilm on dentin sections for analysis
[42]. Time of tested materials application was done in accordance to clinical
resemblance and it is expected to affect the results of the present study. Nano punica granatum herbal extract medicament (gel/cream) and Rose
Bengal dye irrigation (Photodynamic therapy) could be considered potent
antibiofilm strategies for disinfection of the root canal system. 1.
Rocas I and Siqueira J.
Identification of bacteria enduring endodontic treatment procedures by a
combined reverse transcriptase-polymerase chain reaction and re verse-capture
checkerboard approach (2010) J Endod 36: 45-52. https://doi.org/10.1016/j.joen.2009.10.022
2.
Torabinejad M, Handysides R, Khademi
A and Bakland L. Clinical implications of the smear layer in endodontics: a
review (2002) Oral Surg Oral Med Oral Pathol Oral Radiol Endod 94: 658-66.
https://doi.org/10.1067/moe.2002.128962
3.
Kayaoglu G and Ørstavik D.
Virulence factors of Enterococcus faecalis: relationship to endodontic disease
(2004) Crit Rev Oral Biol Med 15: 308-320. https://doi.org/10.1177/154411130401500506
4.
Stewart P and Costerton J.
Antibiotic resistance of bacteria in biofilms (2001) Lancet 358: 135-138. https://doi.org/10.1016/s0140-6736(01)05321-1
5.
Waltimo T, Trope M, Haapasalo M
and Ørstavik D. Clinical efficacy of treatment procedures in endodontic
infection control and one year follow-up of periapical healing (2005) J Endod 31:
863-866. https://doi.org/10.1097/01.don.0000164856.27920.85
6.
Peters O, Laib A, Gohring T and
Barbakow F. Changes in root canal geometry after preparation assessed by high-resolution
computed tomography (2001) J Endod 27: 1-6. https://doi.org/10.1097/00004770-200101000-00001
7.
Estrela C, Estrela CR, Barbin EL,
Spano JCE, Marchesan AM, et al. Mechanism of action of sodium hypochlorite
(2002) Braz Dent J 13: 113-117. https://doi.org/10.1590/s0103-64402002000200007
8.
Lee M, Winkler J, Hartwell G, Stewart
J, Caine R, et al. Current trends in endodontic practice: emergency treatments and
technological armamentarium (2009) J Endod 35: 35-39. https://doi.org/10.1016/j.joen.2008.10.007
9.
Siqueira JF Jr and Lopes HP.
Mechanisms of antimicrobial activity of calcium hydroxide: a critical review
(1999) Int Endod J 32: 361-369. https://doi.org/10.1046/j.1365-2591.1999.00275.x
10. Haapasalo
HK, Siren EK, Waltimo TM, Òrstavik D and Haapasalo MPP. Inactivation of local
root canal medicaments by dentine: an in vitro study (2000) Int Endod J 33:
126-131. https://doi.org/10.1046/j.1365-2591.2000.00291.x
11. Hamblin
M and Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious
disease (2004) Photochem Photobiol Sci 30: 436-450. https://doi.org/10.1039/b311900a
12. George
S and Kishen A. Influence of photosensitizer solvent on the mechanisms of
photoactivated killing of Enterococcus faecalis (2008) Photochem Photobiol 84:
734-740. https://doi.org/10.1111/j.1751-1097.2007.00244.x
13. Cushing
B, Kolesnichenko V and OConnor C. Recent advances in the liquid-phase syntheses
of inorganic nanoparticles (2004) Chem Rev 104: 3893-3946. https://doi.org/10.1021/cr030027b
14. Farmahan
H. Pomegranate, in Recent Trends in Horticulturein the Himalayas (2004) India. 15. Negi
GJ and Jena B.
Antimicrobialactivities of pomegranate, in Pomegranates: Ancient Roots to
Modern Medicine (2006) CRC Press, USA. 16. Haapasalo
M and Ørstavik D. In vitro infection and disinfection of dentinal tubules
(1987) J Dent Res 66: 1375-1379. https://doi.org/10.1177/00220345870660081801
17. Gregorio
C, Arias A, Navarrete N, Rio V, Oltra E, et al. Effect of Apical Size and Taper
on Volume of Irrigant Delivered at Working Length with Apical Negative Pressure
at Different Root Curvatures (2013) J Endod 39: 119-124. https://doi.org/10.1016/j.joen.2012.10.008
18. Tay
C, Quah S, Lui J, Yu V and Tan K. Matrix Metalloproteinase Inhibitor as an
Antimicrobial Agent to Eradicate Enterococcus
faecalis Biofilm (2015) J Endod 41: 1-6. https://doi.org/10.1016/j.joen.2015.01.032
19. Kishen
A. Advanced therapeutic options for endodontic biofilms (2010) Endod Topics 22:
99-123. https://doi.org/10.1111/j.1601-1546.2012.00284.x
20. Stojicic
S, Shen Y and Haapasalo M. Effect of the source of Biofilm Bacteria, Level of
Biofilm Maturation, and Type of Disinfecting Agent on the susceptibility of
Biofilm Bacteria to Antibacterial agents (2013) Endod 39: 473-477. https://doi.org/10.1016/j.joen.2012.11.024
21. Stoodley
L and Stoodley P. Evolving concepts in biofilm infections (2009) Cell Microbiol
11: 1034-1043. https://doi.org/10.1111/j.1462-5822.2009.01323.x
22. Reddy
M, Gupta S, Jacob M, Khan S and Ferreira D. Antioxidant, antimalarial and
antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic
acids from Punica granatum L (2007)
Planta Medica 73: 461-467. https://doi.org/10.1055/s-2007-967167
23. Cavallito
CJ and Bailey J. Allicin, the Antibacterial Principle of Allium sativum:
Isolation, Physical Properties and Antibacterial Action (1944) J Am Chem
Society 66: 1950-1951. https://doi.org/10.1021/ja01239a048
24. George
S, Hamblin M and Kishen A. Uptake pathways of anionic and cationic
photosensitizers into bacteria (2009) Photochem Photobiol Sci 8: 788-795. https://doi.org/10.1039/b809624d
25. Dahl
T, Midden W and Neckers D. Comparison of photodynamic action by Rose Bengal in
gram-positive and gram-negative bacteria (1988) Photochem Photobiol 48: 607-612.
https://doi.org/10.1111/j.1751-1097.1988.tb02870.x
26. Guo
Y, Rogelj S and Zhang P. Rose Bengal-decorated silica nanoparticles as
photosensitizers for inactivation of gram-positive bacteria (2010) Nanotechnol 21:
651-659. https://doi.org/10.1088/0957-4484/21/6/065102
27. Arite
E, Marle J and Cate J. Conofocal microscopy study of undisturbed and
chlorhexidine-treated dental biofilm (2001) J Dent Res 80: 1436-1440. https://doi.org/10.1177/00220345010800051001
28. Zapata
R, Bramante C, Moraes I, Bernardineli N, Gasparoto T, et al. Confocal Laser
Scanning Microscopy Is Appropriate to Detect Viability of Enterococcus faecalis in Infected Dentin (2008) J Endod 34: 1198-1201.
https://doi.org/10.1016/j.joen.2008.07.001
29. Shen
Y, Qian W, Chung C, Olsen I and Haapasalo M. Evaluation of the effect of two
Chlorhexidine preparations on biofilm bacteria in vitro: a three-dimensional
quantitative analysis (2009) J Endod 35: 981-985. https://doi.org/10.1016/j.joen.2009.04.030
30. Shen
Y, Stojicic S, Qian W, Olsen I and Haapasalo M. The synergistic antimicrobial
effect by mechanical agitation and two chlorhexidine preparations on biofilm
bacteria (2010) J Endod 36: 100-104. https://doi.org/10.1016/j.joen.2009.09.018
31. Shoko
T, Soichi T, Megumi M, Eri F, Jun K, et al. Isolation and identification of an
antibacterial compounds from grape and its application to food (1999) Nippon
Nogeikagaku Kaishi 73: 125-128. 32. Webster
P, Webster S, Rich K and McDonald K. Ultrastructural preservation of biofilms
formed by non-typeable Hemophilus influenza (2004) Biofilms 1: 165-182. https://doi.org/10.1017/s1479050504001425
33. Romero
R, Schaudinn C, Kusanovic J, Gorur A, Gotsch F, et al.
Detection of a microbial biofilm in intraamniotic infection (2008) Am J Obstet
Gynecol 198: 135-135. https://dx.doi.org/10.1016%2Fj.ajog.2007.11.026
34. Diaspro
A. Confocal and two -Photon Microscopy: Foundations, Applications, and Advances
(2002) Wiley-Liss press, United States. 35. Gulube
Z and Patel M. Effect of Punica granatum
on the virulence factors of cariogenic bacteria Streptococcus mutans (2016) Microb Pathog 98: 45-49. https://doi.org/10.1016/j.micpath.2016.06.027
36. Irshad
R, Tahir K, Li B, Ahmad A, Siddiqui A, et al. Antibacterial activity of
biochemically capped iron oxide nanoparticles: A view towards green chemistry
(2017) J Photochem Photobiol B 170: 241-246. https://doi.org/10.1016/j.jphotobiol.2017.04.020
37. Stuart
CH, Schwartz SA, Beeson TJ and Owatz CB. Enterococcus
faecalis: its role in root canal treatment failure and current concepts in
retreatment (2006) J Endod 32: 93-98. https://doi.org/10.1016/j.joen.2005.10.049
38. Evans
M, Davies JK, Sundqvist G and Figdor D. Mechanisms involved in the resistance
of Enterococcus faecalis to calcium
hydroxide (2002) Int Endod J 35: 221-228. https://doi.org/10.1046/j.1365-2591.2002.00504.x
39. Soukos
N, Socransky S, Mulholland S, Lee S and Doukas AG. Photomechanical drug
delivery into bacterial biofilms (2000) Pharm Res 17: 405-409. 40. Shen
Y, Stojicic S, Qian W, Olsen I and Haapasalo M. The synergistic antimicrobial
effect by mechanical agitation and two chlorhexidine preparations on biofilm
bacteria (2010) J Endod 36: 100-104. https://doi.org/10.1016/j.joen.2009.09.018
41. Kishen
A, Upadya M, Tegos G and Hamblin M. Efflux pump inhibitor potentiates antimicrobial
photodynamic inactivation of Enterococcus
faecalis biofilm (2010) Photochem Photobiol 86: 1343-1349. https://doi.org/10.1111/j.1751-1097.2010.00792.x
42. Stojicic
S, Shen Y, Qian W, Johnson B and Haapasalo M. Antibacterial and smear layer
removal ability of a novel irrigant, QMiX (2012) Int Endod J 45: 363-371.
https://doi.org/10.1111/j.1365-2591.2011.01985.x Mohamed Ahmed Wakwak, Lecturer of Operative
Dentistry, Faculty of Dental Medicine, Al-Azhar University, Makram Ebid, Nasr
City, Egypt, E-mail: drwakwak2006@azhar.edu.eg Farghaly MA and Wakwak AM. Confocal
laser scanning electron microscope assessment of different antibiofilm
strategies of root canal system disinfection (an experimental study) (2019)
Dental Res Manag 3: 68-73.Confocal Laser Scanning Electron Microscope Assessment of Different Antibiofilm Strategies of Root Canal System Disinfection (An Experimental Study)
Ahmed Mostafa Farghaly
and Mohamed Ahmed Wakwak
Full-Text
Introduction
Materials and Methods
Sterilization of the samples
Preparation of the microorganisms
Confirmation of multispecies biofilm formation


Preparation
of irrigant solutions and medicaments

Grouping
of the samples
Evaluation
using confocal laser scanning microscopy
Statistical
analysis
Results





Discussion
Conclusion
References
*Corresponding author
Citation
Keywords