Selection of Animals for Preclinical Studies
The selection of the type and the number of animals used for the preclinical studies is one of the most important steps. The choice of the species is based on the fact that which one will give the best correlation to the human trials. Generally, experiments are first performed on the rodents and then on the larger animals like canines. Differences in the gut, enzyme activity, circulatory system, or other considerations make certain models more appropriate based on the dosage form, site of activity, or noxious metabolites. For example Canines may not be good models for solid oral dosage forms because the characteristic carnivore intestine is underdeveloped compared to the omnivores and gastric emptying rates are increased. Also, rodents cannot act as models for antibiotic drugs because the resulting alteration to their intestinal flora causes significant adverse effects.
Depending on drugs functional groups, it may be metabolized in similar or different ways between species, which will affect both efficacy and toxicology [1]. Differences in drug response due to species differences are taken into account while extrapolating the data to humans. For Example: Amphetamine and Ephedrine are predominantly metabolized by oxidative deamination in men and rabbits where as in rats aromatic oxidation is the major route.
Most studies are performed in larger species such as dogs, pigs and sheep which allow for testing in a similar sized model as that of a human. In addition, some species are used for similarity in specific organs or organ system physiology. For Example: Swine for dermatological and coronary stent studies, goats for mammary implant studies, dogs for gastric studies, rabbits for dermatological studies etc. (Figure 2) (Table 1)
Stages of Preclinical Studies
Preclinical clinical studies require the time span of 3-6 years. The reason is that, the newly discovered compound is tested at various levels to ensure its complete safety in each and every aspect in animals so that it can be further utilized for testing in humans. Thus the preclinical studies are performed at different stages which are as follows:
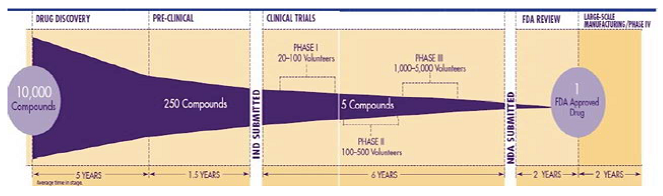
Figure 1: Reduction in number of compounds in each stage of the drug discovery.

Figure 2: The pancreas of the rabbit is removed.
General Observational Tests
It is not possible to devise a scheme of testing which will reveal all the different types of potentially useful pharmacological activity which may be possessed by a particular substance [2]. The person in charge of a screening program must decide on the precise procedure which is to be adopted in individual cases. Much can be learnt by making simple observations on the effect of the compound on the behavior of conscious animals. One of the most useful and best known methods of obtaining the maximum amount of information from these simple tests is that designed by Irwin commonly called as the “Irwin’s Primary Tests for Pharmacological Activity.” In this mice are given intraperitoneal injections of the substance
under test and its effects are assessed by systematic observation of the animal’s subsequent behavior and responses. The observations enable an activity profile to be built up so that the actions of the compound being tested can be compared with that of reference drugs of known activity. Most of the observations listed in the Irwin’s tests are simple and are as follows: (Figure 3) When a drug’s profile is being drawn by the application of Irwin’s Method, small groups of mice are given the drug at a number of dose levels, a useful range is 1, 3, 10, 30 and 100 mg/ kg of body weight but with very active substances it may be given to establish thresholds of activity by reducing the dose in progressive steps below the level 1 mg/100 gms of body weight. Pharmacological activity detected by these simple tests must of course be thoroughly investigated by the methods discussed in the later part of the section.
Screening Tests: The word “screen” implies that the substances under test are exposed to a process which will hold back substances of potential value and let through the rest. In order to achieve this satisfactorily, the mesh of the screen should be small enough to retain all compounds of interest, even if some have to be rejected when they are subjected to the more rigorous procedures which constitute the secondary screening tests [3]. The screening is thus defined as “The process of filtering among the large number of compounds, the one with required biological activity”. The screening program is basically of three types:
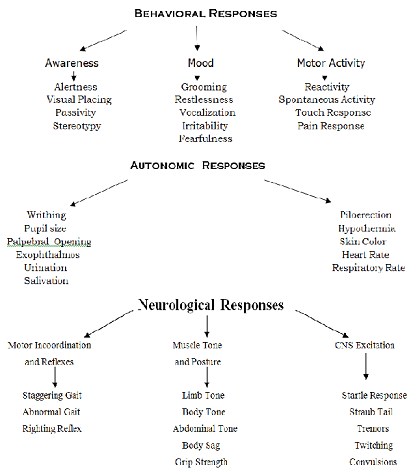
Figure 3: Irwins Primary Tests for Pharmacological Activity.
Simple or Single Screening: This employs a single test or perhaps two similar tests to find substances that are active in a single way. A hypoglycemic test, which measures the ability of a compound to diminish the concentration of sugar in the blood, is an example of this type. This is usually done when the new compound have been produced by a simple modification of the structure of a substance of proved therapeutic value.
Programmed or rational screening: This employs sound physiological, biochemical, pathological knowledge and identification of drug action. The compound is aimed at mitigating the derangement caused by the disease. Use of levodopa in Parkinson based on the finding that the condition resulted from the deficiency of dopamine in the striatum.
Blind or random screening: When a compound of entirely new type comes into the hands of the pharmacologist, it has to be subjected to a series of simple tests designed to reveal the nature of any pharmacological activity it possess and thus to indicate the direction to be taken by more detailed investigations if these seems to be justified. For obvious reasons, the application of this battery of simple tests is described as the blind screening. If the pharmacological activity of a particular kind is discovered in the course of a blind screening program, it will be necessary to investigate it in more detail by one of the methods employed for the study of that particular activity In practice, the overall strategies which are adopted for the pharmacological investigation of new compounds do not differ greatly from one another. Thus, if a substance has been produced for obtaining the particular type of activity, it will still be necessary, when the presence of the primary activity has been confirmed, to apply the test for other pharmacological effects.
Tests on Animal Models of Human Disease
An animal model is a living, non-human animal used during the research for investigation of human disease or for determining the therapeutic potential of a newly discovered compound for the purpose of better understanding the disease and its cure without the added risk of causing harm to an actual human being during the process. The animal chosen will usually meet a determined taxonomic equivalency to humans, so as to react to disease or its treatment in a way that resembles human physiology as needed. Many drugs, treatments and cures for human diseases have been developed with the use of animal models. The animal models used can be classified as follows:
Chemical models: This involves the use of some chemical that selectively destroys some cells or tissues or induce certain pathophysiological condition very similar to what actually occurs in some disease to study the therapeutic effect of a compound. For example: MPTP model for Parkinsonism, where 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is used for the induction of parkinsonism.
Physical models: This involves the use of various physical factors to induce the symptoms similar to that occurring in a disease or disorder. For example: Use of Electric shock to induce convulsions in rats for studying the anticonvulsant activity of a compound.
Surgical models: This involves the surgical removal of certain tissue or organ of the body to study the particular activity of a compound. For example: The pancreas of the rabbit is removed to induce diabetes and to study the antidiabetic effect of a compound.
Genetic models: Genetic manipulation either by addition of genes (Transgenic Technology) or by modification of the existing genes (Gene Knockout Technology) is a very powerful tool with tremendous applications in the drug discovery [4]. Transgenic mouse is becoming very popular animal for studying the disease processes and testing of the newer drugs. It is relatively easy to manipulate the mouse gene. Addition of genes or the loss of gene activity often causes changes in a mouse’s phenotype, which includes appearance, behavior and other observable physical and biochemical characteristics. Screening of bioactive molecules for drug research is one of the important areas where transgenic and knockout mice have tremendous potential. This technology is also being used to create models of human diseases. Some of the newer variations of knockout and transgenic technologies such as knockins (to replace a gene in the genome with a modified version of itself) and the floxP system (to disrupt a gene at a specific time of development) allow investigators to manipulate the gene functions in particular tissues or at certain times. For example: ob/ ob knockout mice model to study obesity and db/db knockout mice model to study diabetes. Table 2
Bioassays and Confirmatory Tests
Bioassays are the procedures by which the potency or the nature of the substance is estimated by studying its effects on living matter. They are usually designed to measure the relative potency of the two preparations, usually a standard and an unknown. Use of standard substance for comparison also helps in solving problems arising from biological variations. The observed response or effect of the unknown would be always relative to the effect that is produced by a standard substance. The standard substance is a pure substance and, in official bioassays it refers to pharmacopoeial standards. In case of hormones, biological products and vaccines it is often necessary to establish the standard response of the standard substances against which unknown samples can be calibrated. Bioassays are also essential in the development of new drugs. In the preclinical assessment of a new compound, the biological activity is compared with that of known (standard) compound using appropriate test systems. In such studies, the tests must be simple reproducible and economical. Biological assessments of a new compound generally consists of carrying out a battery of such assays and based on these tests, constructing a profile of activity. Clinical testing of drugs is guided by such profile of activity generated in animals [5]. Bioassays are usually employed when:
1. A chemical assay for the substance is not available or the substance gets inactivated by interacting with chemicals as in case with hormones.
2. When the quantity of sample is too small.
3. To estimate the concentration of the active principles present in the tissue extracts, the endogenous mediators like acetylcholine, serotonin, prostaglandins etc.
4. To measure the pharmacological activity of new or chemically unidentified substances.
5. To measure the compound’s toxicity.
6. When the bioassay is more sensitive than the chemical assay.
Bioassays are usually done using
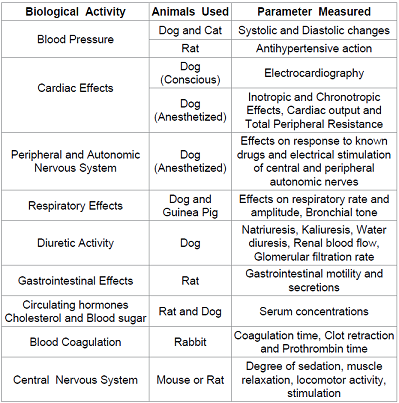
Isolated tissues or organs: The tissues or organs separated from some animals can be used to study some specific activity of the compound. The type of animal and tissue to be selected depends upon the type of activity to be tested. Table 3
Cell cultures: In these assays, the pure cells of specific lines are grown on a suitable culture media and then these pure cell lines are used to measure the specific type of activity. This type of study is commonly employed for measuring the antimicrobial activity of a particular compound against specific microbe, where the pure cultures of microbe are grown on specific culture media and then the antimicrobial drug’s effect is evaluated by measuring the diameter of the zone of inhibition. For example: i) Pure cultures of Mycobacterium megmatis is used for the assay of the antibiotic Bleomycin sulphate. ii) Primary cell culture of mouse spinal cord neurons are used in neuropharmacology for studying neurotransmitter receptor functions.
Intact animals: In these types of assays, the whole animal is used to measure the potency of the compound. These types of assays are less common and are employed for very few substances. For example: Bioassay of insulin using mouse or of digitalis in guinea pigs. Compounds that are found active in the above tests are then taken up for the more detailed and elaborative study for confirming the activity in the more precise manner. In this stage the compounds undergo testing by a large number of more detailed tests for the particular activity and simultaneously for the other analogous activities it possess.
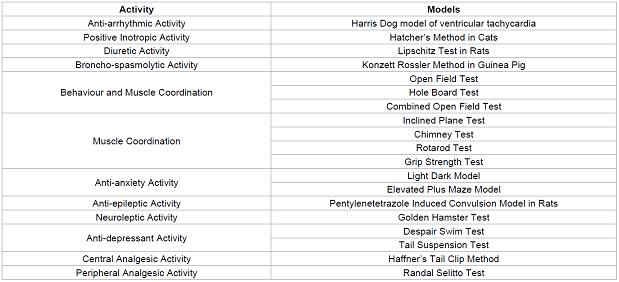
Table 2: Some of the most commonly used animal models for studying various activity.
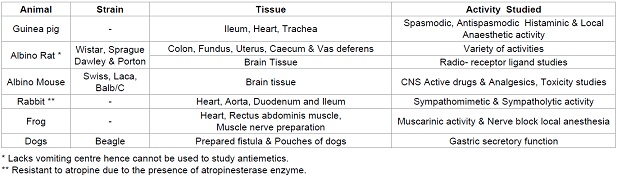
Table 3: Some of the animal tissues used and the activity.
Determination of mechanism of action and systemic pharmacology
In most of the cases where the new compound has been obtained via rational approach, combinatorial chemistry, molecular modeling or sometimes even in case of chemically synthesized compounds, the mechanism of action of compound is predetermined as the compound is synthesized with the predetermined target in mind hence the mechanism of action is already known. However in case of compounds obtained from natural sources like plant extracts, microbes etc. the determination of mechanism of action and the targets of their action is required which is a troublesome task. Radio ligand Binding Assays are the most used techniques for this purpose which involves the use of radioactive atom, bound to the new compound and then its fate inside the biological system is studied using radio-detection techniques. Recently, newer approaches with more precise measurements are employed for this purpose and some of them are enumerated as follows:
1. Fluorescence Resonance Energy Transfer (FRET)
2. Fluorescence Correlation Spectroscopy (FCS)
3. Homogeneous Time Resolved Fluorescence Technology
(HTRFT)
4. Confocal Microscopy
5. Scintillation Proximity Assay (SPA) and many more.
Irrespective of the primary mechanism of compound’s action, its effect on other vital organs of the body like brain, heart, kidney, liver, respiratory organs, gastrointestinal tract etc are simultaneously determined by measuring related parameters and the complete pharmacological profile of the compound is explored.
Quantitative Tests: Quantification of the compound’s response or activity is very essential as it is the dose that makes a compound a drug or a poison. There are basically two types of measurement whereby the effect of a compound on an animal may be evaluated:
i) Graded Effect: These are based on the principle that there is a proportionate increase in the observed response with a subsequent increase in the concentration or the dose. For example: The contraction of the smooth muscle preparation for assaying histaminic activity of a compound.
ii) Quantal Effect: These are based on “All or None” phenomenon. This type of response of a group of animals is measured in order to determine the percentage responding. In these cases the end point is an all or none response. For example: Whether a compound causes cardiac arrest or not. One of the most important parameter that is quantified by these studies is the ED50 value. ED50 is defined as the dose effective for producing a certain sign in 50% of the animals of a group. The units are those of the dose (mg/kg) and the value is, of course, different for each route of administration. The ED50 is calculated, since it would be fortuitous that one of the doses of a series should produce the effect in exactly half of the animals. When the all or none response, also called the quantal response is death, the ED50 becomes the LD50 or the lethal dose for 50% of the animals. Sometimes the ED75, the ED10 and the ED99 etc, for example, are desired in order to know a dose affecting most of the animals, a nearly minimally effective dose etc. There are two types of methods for calculating ED50:
i) Graphical Method: Miller and Tainter Method, Litchfield and Wilcoxon Method.
ii) Arithmetical Method: Reed and Muench Method, Karber
Method.
Determination of Pharmacokinetic Parameters
Pharmacokinetics is the quantitative study of the drug movement in, through and out of the body with respect to time. This is the one of the most important stage as it is found that maximum percentage of compounds are rejected in preclinical studies during this stage only due to undesirable pharmacokinetic profile. The following parameters are determined during pharmacokinetic studies:
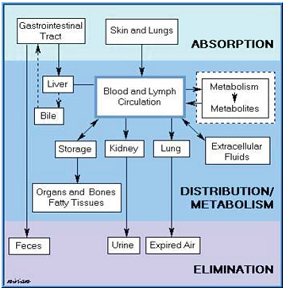
Figure 4: Pharmacokinetic profile.
1. Absorption Parameters:
a) Absorption of compound via various routes.
b) Mechanism of absorption.
c) Various factors affecting absorption.
d) Rate and extent of absorption (Bioavailability).
2. Distribution Parameters:
a) Tissue permeability of compound.
b) Volume of distribution.
c) Protein and tissue binding of compound.
d) Factors influencing distribution.
3. Metabolic Parameters:
a) Pathways of metabolism.
b) First Pass Metabolism.
c) Factors influencing metabolism.
d) Enzyme induction / Enzyme inhibition.
e) Bioactivation and other parameters.
4. Excretion Parameters:
a) Routes of excretion.
b) Clearance.
c) Dose Adjustments.
d) Factors influencing excretion.
5. Interaction:
a) Pharmacokinetic interactions.
b) Pharmacodynamic interaction.
Other Parameters:
a) Plasma Concentration – Time profile.
b) Bioavailability studies.
c) Therapeutic Concentration Range studies.
d) Design of dosage regimen.
e) Concentration response studies.
Toxicity Studies
It involves the study of injurious effects of the compound on the animals along with the mechanisms of toxicity. The main aim of these studies is to determine the safety of the compound in at least 2 animal species, mostly mouse/rat and dog by oral and parenteral routes. Table 4 It is during the toxicity studies when the multiple dose levels are selected and assayed in order to determine NOAEL (No Observable Adverse Effect Level) value, which will help to determine a safe starting dose level and dose escalation scheme for the phase I clinical trials.
Regulatory Bodies involved in Preclinical Trials
(Figure 5)
The regulation of the Drug Discovery process begins from the preclinical stage where the large number of regulatory authorities has established various well defined protocols that are to be complied during the entire process. GLP (Good Laboratory Practices) is one of the essential requirements of all modern testing facilities. It embodies a set of principles that provide a framework within which laboratory studies are planned, performed, monitored, reported and archived. In order to promote international cooperation and harmonization, the Organization for Economic Cooperation and Development (OECD) has developed certain test guidelines and principles of GLP. GLP provides an assurance to regulatory authorities that the data submitted are a true reflection of the results obtained during the study and can, therefore, be relied upon when making risk/safety assessment. GLP are a useful set of standards for any research laboratory wishing to standardize procedures and verify the reliability of data produced. This is an issue of increasing importance to the research sponsors. In 1980, OECD Council recommended that all the member countries should apply these guidelines in the testing of compounds and the data generated in accordance with the OECD test guidelines and OECD principles of GLP shall be accepted by all the member countries. Some other regulatory bodies involved are: Table 5
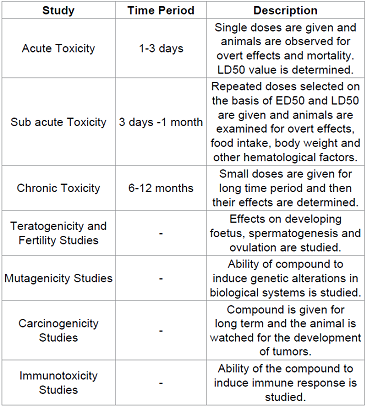
Table 4: Various types of toxicity studies that are done during preclinical studies.

Table 5: Some other regulatory bodies involved.
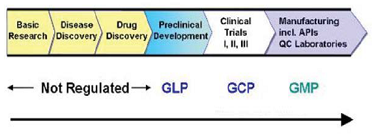
Figure 5: Preclinical Studies are conducted as per GLP requirements as detailed in 21 CFR Part 58.
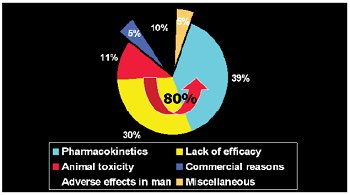
Figure 6: Statistics regarding Preclinical Trials.
Statistics regarding Preclinical Trials
• Approximately 10% of total cost involved (15-30 Billion Dollars) is spent for preclinical studies during drug
discovery process [6].
• Approximately 80% of the compounds taken for preclinical
study fail during the process.
• Approximately 39% of the compounds are rejected in the preclinical studies due to poor pharmacokinetic profiles.
• Approximately 11% are rejected due to toxicological issues.
Figure 6
Conclusion
Preclinical Studies though complicated and time consuming, acts as the bridge between the synthesis or discovery of a newly discovered compound and its testing is human. The data generated during preclinical study helps a lot in approximately picking up a right compound, a right formulation and a right drug delivery system that reduces the risk of injurious effects on humans. However there are certain shortcomings of preclinical studies like there is limited information on mechanism of action, biologically relevant animal species or models of disease are not always available and sometimes limited information is available to support the validity of extrapolation from animal to humans. Still preclinical studies play a very important role in the drug discovery process.
References
1.
Ariens EJ, Simonis AM, Offermeier J. Introduction to General Toxicology (1976)
Academic Press Inc, USA.
2.
Crossland J. Lewis’s Pharmacology 4th edn (1971) Williams and Wilkins Co., USA.
3.
Turner RA, Peter Hebborn. Screening Methods in Pharmacology (1965) Elsevier,
USA.
4.
Vogel HG. Drug Discovery and Evaluation 2nd edn (2002) Springer, USA.
5.
Kulkarni S.K, Practical Pharmacology and Clinical Pharmacy 1st edn (2008)
Vallabh Publications, India.
6.
Barile FA. Principles of Toxicological Testing (2008) CRC Press, USA.
*Corresponding author
N Venkatesan, S.B College of Pharmacy, Anaikuttam Road, Anaikuttam, Sivakasi, India E-mail: nvenkatpharmabhu@gmail.com
Citation
N.Venkatesan, M. Ramanathan (2017) Preclinical Toxicity Studies-Tool of Drug Discovery. PVPE 1: 1-7
Keywords
Drug Discovery, Therapeutic Concentration, Pharmacokinetics, Spectroscopy, antimicrobial activity


 PDF
PDF