Introduction
Mesua Ferrea L . commonly known as Nagkesar belongs to family Calophyllaceae . The plant is used medicinally in various ailments [ 1 ] . The decoction of seeds of M . ferrea is given for the treatment of gastritis, bronchitis and to cure snake bite . Leaves of M . ferrea are antidote for snake bites and scorpion sting [ 2 ] . The different extracts of plants have shown anti -ulcer, anti venom, anti protozoal, anti cancer, anti - oxidant activities [ 3 ] . The present study reports the isolation and structural elucidation of two compounds isolated from the seeds of Mesua Ferrea L (Figure 1) .
Experimental
General procedures
Melting points ( MP ) are uncorrected . 1 H NMR was recorded on 300 MHz Varian XL spectrometer, 13 C NMR spectra were recorded on Varian XL 75 MHz spectrometer, IR spectra were recorded in KBr disk on Perkin Elmer - 377 spectrometer, EIMS on Jeol - JMS D 300 mass spectrometer [ 4 ] . A ll chemical shifts (d) are given in ppm and Me 4 Si was used as internal standard . The carbon type (CH 3 , CH 2 ,CH) was determined by DEPT experiments . Chemicals are of analytical - reagent grade and column chromatography was carried out on alumina grade III and TLC on silica gel G (CDH/Glaxo laboratories) . Spots were visualized by exposure to iodine vapor or by spraying with H 2 SO 4 - vanillin solution followed by heating at 105 _C for 5 min .
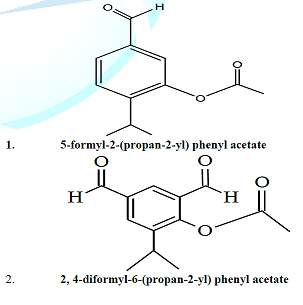
Figure 1: Chemical structures of compound1-2.
Plant material
The seeds (10 kg) of M . ferrea L . were collected from the market of Ujjain city and were identified by the aut horities of the Institute of Environment Management and Plant Science, Vikram University, Ujjain . A voucher specimen was deposited in the herbarium of the School of Studies in Botany, Vikram University, Ujjain, India .
Extraction and isolation
The seeds ( 10kg) were shade dried, cleaned, coarsely powdered and extracted with hexane in soxhlet - extractor for 72 h . The extract was concentrated by rotary evaporator to afford solid mass (265 mL) . Usual work up yielded (32g) solid extract which was separated by re peated column chromatography on alumina grade III . The column was eluted by gradient elution in increasing order of polarity like hexane, benzene, EtOAc and methanol . The fractions were collected in bulk and monitored by TLC . The residue (6 . 8 g) of hexane fraction was rechromatographed on alumina on the basis of increasing order of polarity of eluents . A well - stirred suspension of alumina III (100 – 150 g in petroleum ether 60 – 80) was poured into the column (150 cm long and 50 mm in diameter) . When the absorb ent was well settled, the excess of hexane was allowed to pass through the column. With silica gel in hexane, the mass was made into slurry and digested in a well stirred column. The column was successively eluted with the hexane, benzene, EtOAc and meth anol and their mixtures of increasing polarity . The column hexane fraction (Fr. No. 1 - 10) afforded one compound in pure form designated as A23, column of hexane: benzene (1:1, v/v, Fr . No. 28 - 34) afforded another compound in pure form designated as A24.
Screening of Anticancer Activity
The anticancer activities of the compound 1 and 2 of M . ferrea L . (Seeds) [ 5 ] against the three Cancerous cell lines were undertaken by using MTT (3 - (4, 5 - dimethylthiazol - 2 - yl) - 2 ,5 - diphenyltetra zolium bromide ) assay . The th ree tested cancer cell lines were Hek (Human embryonic kidney cell lines), IMR - 32 (Human neuroblastoma) and C6 (Rat glioblastoma) . All the three cell lines were maintained in DMEM (Media) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic (Pe ncillin/ Streptomycin) [ 5 ] . All the cell lines were cultured in 75cm 2 T - flask and maintained at 37 0 C in 5% carbon dioxide humidified incubator for 24 hours . The MTT Assay is based on the protocol illustrated by Mosmann [ 6 ] and was executed in 12 - well flat bottom plates . The varying concentrations of compounds 5, 10, 15, 20, 25, 30, 35, 40, 45, 50 and 55 μg/ml were prepared by a serial dilution method . After 24 hours 300 μl of MTT solution and PBS (Phosphate buffer saline) was added to all the wells and incubated for 3 hours in a 5% CO 2 humidified incubator . 300 μl of DMSO (Solubilization buffer) were added to each well and incubated for 10 minutes. The absorbance of each well was determined using a microplate reader at 550 nm. A graph of cell viability versus concentrations was plotted for each compound. The half maximal inhibitory concentration (IC 50) values were obtained from the plotted graph . Further dilutions will only be performed on the compounds with IC 50 values less than 15 μg/ml. Three independent experiments were conducted to assure the accuracy of the results.
Compound 1
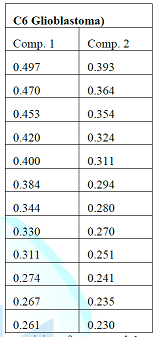
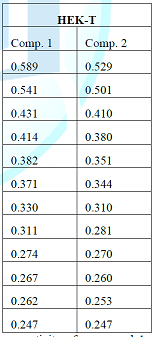
Designation : 5 - formyl - 2 - (propan - 2 - yl) phenyl acetate, Elemental analysis : Calculated for C 12 H 14 O 3 , C= 69 . 88 %, H= 6 . 84 %, O= 23 . 27 %, Observed values C= 69 . 60 %, H= 6 . 94 %, O= 23 . 11 % , Molecular formula : C 12 H 14 O 3 , Solubility : CDCl 3 , Molecular ion peak : M + 206, TLC solvent system : Chloroform: methanol: acetic acid (6:4:0 . 5, v/v), Recrystallization : Chloroform : Methanol, State : Solid, IR spectrum ( ʎ max, KBr, cm - 1 ) 3382, 2967, 1603, 1429, 1216, 1044, 925, 761, 670 cm - 1 , 1 H NMR spectrum (300 MHz, CDCl 3 , TMS, δ) δ 10 . 10 (s, CHO), δ 7 . 69 and 7 . 26 (m, 3H of benzene), δ 2 . 59 (s, - CO - CH 3 ), δ 1 . 26 (d, 2CH 3 of isopropyl), δ 3 . 2 (m, - CH of isopropyl), 13 C NMR spectrum (75 Hz, CDCl 3 , ppm) 133 . 5, 131 . 6, 127 . 1, 141 . 7, 192 . 8, 160 . 0, 22 . 1, 12 . 1 ppm, ESI - MS spectrum (m/z, rel . inte . ) 206 [M + ], 198, 179, 109 . C6 Glioblastoma) Comp . 1 Comp . 2 0 . 497 0 . 393 0 . 470 0 . 364 0 . 453 0 . 354 0 . 420 0 . 324 0 . 400 0 . 311 0 . 384 0 . 294 0 . 344 0 . 280 0 . 330 0 . 270 0 . 311 0 . 251 0 . 274 0 . 241 0 . 267 0 . 235 0 . 261 0 . 230 Table 1: Anticancer activity of compound 1 and 2 of M . ferrea L . (Seeds) against C6 (Rat glioblastoma) . HEK - T Comp . 1 Comp . 2 0 . 589 0 . 529 0 . 541 0 . 501 0 . 431 0 . 410 0 . 414 0 . 380 0 . 382 0 . 351 0 . 371 0 . 344 0 . 330 0 . 310 0 . 311 0 . 281 0 . 274 0 . 270 0 . 267 0 . 260 0 . 262 0 . 253 0 . 247 0 . 247 Table 2 : Anticancer activity of compound 1 and 2 of M . ferrea L . ( Seeds) against Hek (Human embryonic kidney cell lines) .
Compound 2
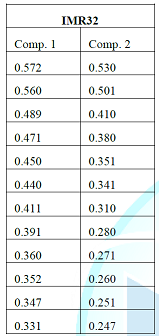
Designation : 2, 4 - diformyl - 6 - (propan - 2 - yl) phenyl acetate, Elemental analysis : Calculated for C 13 H 14 O 4 , C= 66 . 66 %, H= 6 . 02 %, O= 27 . 32 %, Observed values C= 67 . 49 %, H= 6 . 00 %, O= 2 6 . 12 %, Molecular formula : C 13 H 14 O 4 , Solubility : CDCl 3 , Molecular ion peak : M + 234, TLC solvent system : Benzene: ester: acetic acid (9:1:0 . 5), Recrystallization : Chloroform: Methanol, State : Solid, IR spectrum ( ʎ max, KBr, cm - 1 ) 3022, 1638, 1679, 1436, 12 96, 1216, 759, 669, 595, 553 cm - 1 , 1 H NMR spectrum (300 MHz, CDCl 3 , TMS, δ) δ 7 . 26, δ 7 . 93 (2s, 2H of benzene), δ 10 . 27 ( s, Ar - CHO), δ 10 . 48 (s, Ar - CHO), δ 2 . 93 (s, CO - CH 3 ), δ 1 . 24 (d, 2CH 3 ), δ 3 . 34 (m, CH of isopropyl), 13 C NMR spectrum (75 Hz, CD Cl 3 , ppm) 126 . 4, 136 . 3, 136 . 0, 117 . 9, 144 . 1, 190 . 24, 195 . 81, 165, 22 . 1, 12 . 1 ppm, ESI - MS spectrum (m/z, rel . inte . ) 234[M + ], 275, 207, 179, 127 . IMR32 Comp . 1 Comp . 2 0 . 572 0 . 530 0 . 560 0 . 501 0 . 489 0 . 410 0 . 471 0 . 380 0 . 450 0 . 351 0 . 440 0 . 341 0 . 411 0 . 310 0 . 391 0 . 280 0 . 360 0 . 271 0 . 352 0 . 260 0 . 347 0 . 251 0 . 331 0 . 247 Table 3 : Anticancer activity of compound 1 and 2 of M . ferrea L . (Seeds) against IMR - 32 (Human neuroblastoma) .
Results and Discussions
The novel compounds were identified mainly by their IR, 1 H NMR, 13 C NMR and Mass spectrometry analysis.
Compound 1
IR spectrum ( ʎ max, KBr, cm - 1 ) The absorption bands at 3382, 2967, 1429, 1216 cm - 1 were due to the presence of – CH stretching and bending vibrations, while the absorption bands at 761, 862, 1429, 1603 and 1216 cm - 1 indicated the aromatic nature of A23 [ 7 ,8 ] .
1H NMR spectrum (300 MHz, CDCl 3 , TMS, δ) The signal at δ 10 . 10 showed the presence of – CHO group in the molecule. The multiplets at δ 7.69 and δ 7. 26 showed the presence of benzene ring in the molecule. Hence the molecule is aromatic aldehyde . The singlet at δ 2 . 59 was due to acetyl group . Doublet at δ 1.261 was assigned to – CH group and a multiplet at δ 3 . 2 showed the presence of isopropyl group in the molecule [ 9 ] .
13 C NMR spectrum (75 Hz, CDCl 3 , ppm) The 13 CNMR suggests that it consists of 12C skeleton . The peaks at 13.5, 131.6, 127.1, 141.7 ppm were due to benzene nucleus in the molecule . The peak observed at 192.8 ppm was due to – CHO group in the molecule [ 1 0 ] . The carbonyl group of acetyl group was resonated at 160. 0 ppm and the peaks observed at 22 . 1 and12 . 1 ppm were due to methane and methyl carbons of isopropyl group present in the molecule .
ESI - MS Spectrum (m/z, rel . inte . ) The ESIMS showed the molecular ion peak at m/z 206 [M + ] suggesting its molecular formula as C 12 H 14 O 3 . The other fragments were obtained at m/z 198, 179,109 were also consistent with the proposed structure . Thus on the basis of above spectral evidences, the compound was identified and characterized as 5 - formyl - 2 - (propan - 2 - yl) phenyl acetate [ 11 ] .
Compound 2
IR spectrum ( ʎ max, KBr, cm - 1 ) The IR spectrum showed strong bands at 3022 cm - 1 and 1436 cm - 1 due to C - H stretching and C - C ring stretching . The absorption band appeared at 1638 cm - 1 showed the presence of carbonyl group of aldehyde in the molecule . Absorpt ion band at 1679 cm - 1 indicates – O - CO - CH 3 functionality in the molecule . Absorption bands at 1296 and 1216 cm - 1 were due to C - O stretching of aldehyde and carbonyl of acetyl group. The absorption bands at 759,669,595 and 553 cm - 1 were due to out of plane C - H bending [ 1 1 ] .
1H NMR spectrum (300 MHz, CDCl3 , TMS, δ) 1 H NMR spectrum in CDCl 3 showed that it is a naturally acetylated polar aromatic compound having aldehyde group . Two aromatic protons were resonated at δ 7 . 26 - 7 . 93 as a sharp singlet, which indicates that other positions were derivatized natura lly . The peak appeared at δ 10 . 27 as sharp singlet indicates the presence of aldehyde group, in benzene nucleus . The peak appeared at δ 10 . 48 which is deshielded, indicates that two aldehyde groups are present nearby acetoxyl group . The acetyl ( - CO - CH 3 ) methyl group was resonated at δ 2 . 93 as a sharp singlet . It is more deshielded than ketone, which means it is attached as – O - CO - CH 3 . Two methyl groups were resonated at δ 1 . 24 as a doublet and methane proton was at δ 3 . 34 as a multiplet, which indicates that isopropyl group is present in benzene nucleus [ 1 2 ] .
13C NMR spectrum (75 Hz, CDCl 3 , ppm) The 13 C NMR spectrum suggested that it consists of 18C skeleton . The peaks at 126 . 4, 136 . 3, 136 . 0, 117 . 9, 144 . 1ppm were due to benzene nucleus in the molecul e . The peaks observed at 190 . 24 and 195 . 81 were due to aldehydic group in the molecule, one is deshielded (195 . 81) due to the presence of – O - CO - CH 3 group present at ortho - position to it . The carbonyl group of acetyl was resonated at 165 ppm and the peak s observed at 22 . 1 and 12 . 1 ppm suggested that isopropyl group is present in the molecule .
ESI - MS spectrum (m/z,rel . inte ) The molecular ion peak of A24 was found at 234 [M + ] which suggested its molecular formula as C 13 H 14 O 4 . Other abundant fragments w ere obtained at m/z 275,207,179 and 127 were also consistent with the proposed structure . Thus on the basis of above spectr al data compound A24 has been ch aracterized as 2, 4 - diformyl - 6 - (propan - 2 - yl) phenyl acetate [ 1 1 ] .
Screening of Anticancer Activity The novel compounds (1 and 2) of M . ferrea L . (Seeds) showed significantly anticancer activities against Hek (Human embryonic kidney cell lines), IMR - 32 (Human neuroblastoma) and C6 (Rat glioblastoma) . Among the two compounds, com pound 1 showed strong ant icancer activity against the three tested cancer cell lines while as compound 2 showed moderate anticancer activity . The results are being given in Table No . 1, 2 and 3 .
Conclusion
From the survey of the literature to the best of our knowledge the compo unds were novel and are being reported first time by us from seeds of M . ferrea L . and further examination of the constituents of this plant is currently in progress .
Acknowledgements
Authors gratefully acknowledge Natural Product Research Centre (SOS C hemistry) Vikram University Ujjain, CDRI Lucknow and IUST Srinagar (J&K) for providing research infrastructure, use of different techniques like IR, NMR and mass spectra and checking out their anticancer activity .
References
1.
Ambasta SP. The useful plants of India (1994)
Publication and information directorate, CSIR, New Delhi.368.
2. Garg S, Sharma K, Ranjan R, Attri P, Mishra P. In vivo
antioxidant activity and hepatoprotective effects of methanolic extract of
Mesua ferrea Linn(2009)Int J Pharm Tech. Res 1: 1692-1696.
3. Sahni KC. The book of Indian trees(1998) Bombay natural history society,
USA.
4. Ali MA, Sayeed MA, Bhuiyan MSA, Sohel FI, Yeasmin MS. Antimicrobial screening of Cassia fistula and Mesua ferrea (2004) J Med Sci 4:
24-29.
5. Chakraborty DP, Purkayastha M, Bose PK. On the antibiotic properties of some
constituents of Mesua ferrea Linn (1958)N. I. S. Junior Research Fellow
25:8-11.
6. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays (1983) J Immunol Methods 65: 55-63.
7. Bellamy LJ. The Infrared Spectra of Complex Molecules (1975) Chapman and
Hall, UK.
8. Dyer JR. Application of Absorption Spectroscopy of Organic Compounds (1984)
Prentice and Hall of India Ltd 1: 33-38.
9. Jackson LM, Sternhill S. Application of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry
(1969) Pergamon Press,USA 5: 48.
10. Crespo-Ortiz MP, Wei MQ. Antitumor activity of Artemisinin and its derivatives:
from a well known antimalarial agent to a
potential anticancer drug (2012) J. Biomed. And Biotechnol.
11. Harborne JB.Phytochemical Methods: A Guide
to Modern Techniques of Plant Analysis. 3rd Edn. (1998)
Chapman and Hall, UK 1: 129-138,302.
12. Bahl BS,BahlA. A Text Book of Organic Chemistry 13th Edn (1992) Schand and
Company Ltd, India 11-14.
*Corresponding author
Masood Ayoub Kaloo, Department of Chemistry, Islamic University of Science and Technology (IUST), Awantipora Pulwama, India, E-mail: masood.kaloo@islamicuniversity.edu.in
Citation
Faizan DK, Mehta BK, Yasir AL, Masood AK. Novel Phytochemical Constituents Identified from the Seeds of Mesua Ferrea L and their Anticancer Activity (2017) Pharmacovigilance and Pharmacoepidemiology 1: 1-4.
Keywords
Phytochemical, Mesua Ferrea L, Seeds, Calophyllaceae, Anticancer activity


 PDF
PDF