Research Article :
Myalgic encephalomyelitis/chronic fatigue
syndrome (ME/CFS), is a chronic and often disabling disease. Although the exact
pathophysiological mechanism of ME/CFS is unknown, immunological abnormalities
may play an important role. Curcumin is an herb with powerful anti-oxidative
and anti-inflammatory properties. Therefore, we hypothesized that curcumin
would have favorable effects on symptomatology in ME/CFS patients. In an open
trial among 65 ME/CFS participants, 6 stopped the use of curcumin because of
side effects and 8 did not complete the end of study questionnaire. Before and
8 weeks after the use of curcumin complexed with phosphatidyl choline-, 500 mg
bid, participants completed the CDC inventory for assessment of Chronic Fatigue
Syndrome. The CDC questions (n=19) were scored and divided into 2 parts: the
first being specific for CFS complaints (n=9), the second being scores of less
specific symptoms (n=10); denoted as CDC other score. Results showed that 8
weeks of curcumin significantly decreased the CDC CFS-related symptom scores
and CDC other scores, especially in patients with mild disease. Conclusion: in
this open-labeled study 8 week curcumin use in a phosphatidyl choline complex
reduced ME/CFS symptomatology, especially in patients with mild disease severity. Curcumin is an herb with powerful
anti-oxidative, anti-inflammatory,
anti-mutagenic, and anti-microbial properties [1-9]. Oxidative stress can
lead to chronic inflammation. Curcumin can decrease TNF-α production and can inhibit
inflammatory cytokines. See for extensive reviews the studies of Pulido-Moran
[10-20]. Because of these properties, curcumin has been studied in inflammatory
diseases in lungs, joints, bowels, brains, and the cardiovascular system,
rheumatoid arthritis, inflammatory bowel disease, Alzheimers disease, mood
disorders, cancer, diabetes, pain. Although animal Experimental studies are
overwhelmingly positive, there is only limited data from human studies [21-27]. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
(ME/CFS), is a chronic and often disabling disease. The exact prevalence is
unknown but estimates in the US vary between 836.000 and 2.5 million patients,
in the Netherlands between 20.000 and 80.000 patients. Patients with ME/CFS
have been found to be more functionally impaired than those with other
disabling illnesses [28-30]. Although the exact
pathophysiological mechanism of ME/CFS is unknown, immunological abnormalities
may play an important role. Oxidative stress is increased in ME/CFS patients.
The data on cytokines are contradictory and a recent meta-analysis showed no
overall change for approximately 80 plasma cytokines studied in ME/CFS patients
compared to controls. We previously showed in an open treatment trial that
curcumin has favorable effects on symptomatology in
ME/CFS patients, possibly because of the anti-oxidative and anti-inflammatory
properties of curcumin. In the present study we hypothesized that effects in
patient reported outcomes might be dependent on the severity of the disease
[31-37]. Patients with ME/CFS were asked
to participate in the registry and gave informed consent. All fulfilled the
Fukuda criteria for CFS and the ME criteria. Disease severity was scored
according to the ME criteria as mild, moderate, or severe. For this analysis,
patients with moderate and severe disease severity were grouped together and
compared with those who had mild disease [38,39]. Patients were asked to complete
the CDC inventory for assessment of Chronic Fatigue Syndrome, Dutch language
version, prior to the start of using curcumin and return the questionnaire by
mail or email. After 8 weeks of curcumin use, the same questionnaire was
completed. Prior to the start of curcumin they also completed the SF-36
questionnaire [40-42]. Patients used Curcumin Phytosome (Curcuma
longa extract complexed with phosphatidylcholine; NOW®) 500 mg capsules twice a
day. According to the manufacturer the capsules contained at least 18% (90 mg)
curcuminoids. In total 65 patients
participated. Six patients stopped the use of curcumin because of side effects
(all of gastro-intestinal origin). Eight patients did not return the second
questionnaire despite two reminders. These 14 participants were excluded from
the analysis, leaving 51 participants with complete data. No other treatments
for ME/CFS were used during this study period. No side-effects were reported in
the patients who completed the trial period and returned the second
questionnaire. The work described has been carried out in accordance with The
Code of Ethics of the World Medical Association (Declaration of Helsinki) for
experiments involving humans. The medical ethics committee of the Slotervaart
Hospital, Amsterdam, NL, approved the use of patient data for research (code
U/17.089/P1736). The CFS CDC inventory contains 19
symptom questions and collects information about the presence, frequency, and
intensity of 19 illness-related symptoms during the month preceding the
interview; these symptom questions include all the CFS-defining symptoms.
Perceived frequency of each symptom was rated on a five-point scale (0-4) and
severity or intensity of symptoms was measured on a four-point scale (0-3).
Individual symptom scores were calculated by multiplying the frequency score by
the intensity score. For this purpose the intensity scores were transformed
into equidistant scores (0, 1, 2.5, and 4) before multiplication. This results
in a range of 0–16 for each symptom [40]. The CDC score contained the
summed score of the 19 symptoms. For the purposes of this analysis, we
subdivided the CDC score into 2 parts: the CFS score and the CDC other score.
The CFS score consisted of the 8 CFS defining symptoms namely, post-exertional
fatigue, unrefreshing sleep, problems remembering or concentrating, muscle
aches and pains, joint pains, sore throat, tender lymph nodes and swollen
glands and headaches. In the CFS diagnostic criteria memory and concentration
difficulties are taken as one combined symptom, whereas in the CDC inventory
these 2 symptoms are scored separately. Therefore, for the CFS scores 9
symptoms were summed. The 10 remaining CDC questions that were not related to
the CFS score were also summed and labeled as CDC-Other score. The CDC other
score is composed of the symptoms: diarrhea, fever,
chills, sleep problems, nausea, stomach or abdominal pain, sinus or nasal
problems, shortness of breath, light hypersensitivity and
depression. Moreover, in the second, post-curcumin
questionnaire, we asked how you rate your global physical and mental condition
after using curcumin: 0: strongly deteriorated, 1: mildly deteriorated, 2: no
change, 3: mildly improved and 4: strongly improved. One patient reported mild deterioration
after curcumin use. For the chi-square analysis this Patient was added to the
no change group. Similarly, the two improvement answers were also analyzed
together. Scores were tested for normal
distribution using the Shapiro-Wilk test in SPSS (IBM SPSS version 21).
Normally distributed data are presented as mean (SD) and non-normally
distributed data as median (IQR). Data were compared with Students
test for paired and unpaired data, and with the Mann-Whitney U test for
non-normally distributed data, where appropriate. Chi square analysis was
performed on the distribution of the Patient Reported Outcome Measure (PROM):
yes or no change in physical and mental condition after curcumin use. A p value
<0.05 was considered significantly different. Table
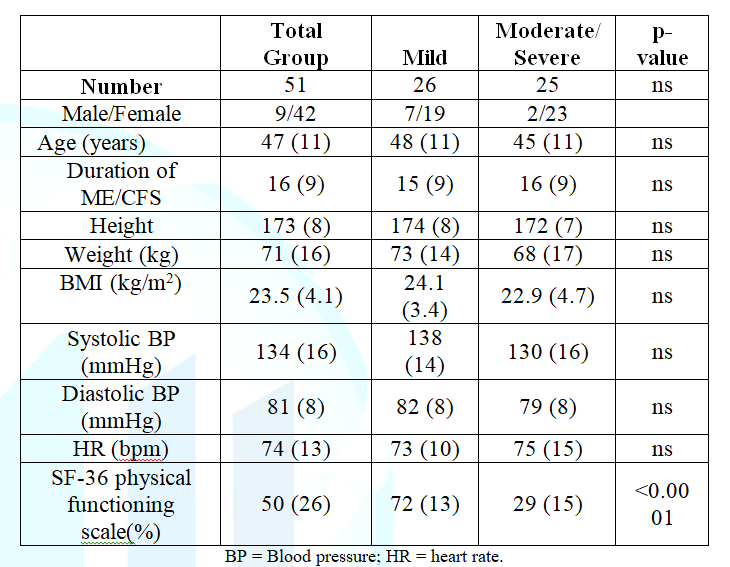
1 shows the demographic data for the 51 participants
analyzed. Forty-two females and 9 males were included. The mean disease
duration was 16 (9) years, and the mean age was 47 (11) years. Except for
height and weight, there were no differences between males and females (data
not shown). According to the ME criteria 26
patients were graded as mild and 25 as moderate to severe (15 moderate and 10
severe patients). Table 1 also shows the baseline data for the mild vs the
combined moderate and severe group. No differences were present except for the
SF-36 physical functioning scale; as expected, mildly affected patients had a
higher physical functioning scale than the moderately and severely affected patients
p<0.0001. Table 1:
Baseline characteristics of the total group and of the mild vs the moderate to
severe ME/CFS patients. Table
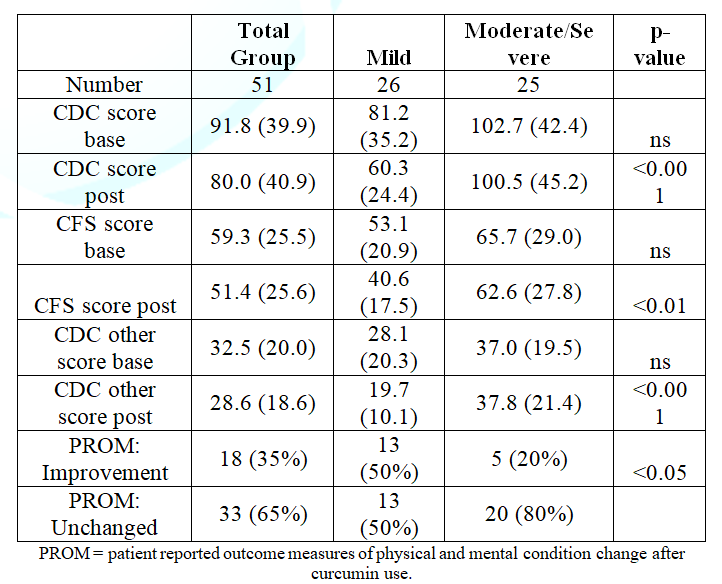
2 shows the CDC inventory score results and the PROM:
the change in physical and mental condition after curcumin use. Table 2:
CDC, CFS and CDC other scores for mild vs moderate/severe ME/CFS patients. Figure
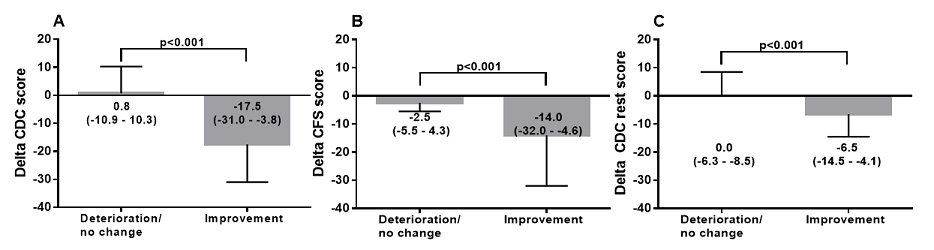
1 shows the data of the PROM: unchanged and improved
physical and mental improvement versus the change in the total CDC score (post
curcumin use minus pre curcumin use). Patients with the PROM: unchanged showed
a significantly lower change in the total CDC score compared to the change in
patients reporting improvement Table 2 also shows the
differences between patients with mild disease vs moderate and severe disease.
Baseline CDC, CFS and CDC other scores were all higher in the mildly affected
patients versus the moderate and severe patients, but did not reach statistical
significance. After 8 weeks of curcumin use, both the total CDC scores, the CFS
scores and CDC-other scores were significantly lower (p<0.001, p<0.01 and
p<0.001 respectively), compared to pre-curcumin use in
the mild ME/CFS group, but were not different in the moderate to severe ME/CFS
group. Figure 1 show the changes in CDC,
CFS and CDC other scores with respect to the patient reported outcome of
neutral/deterioration or improvement. Figure
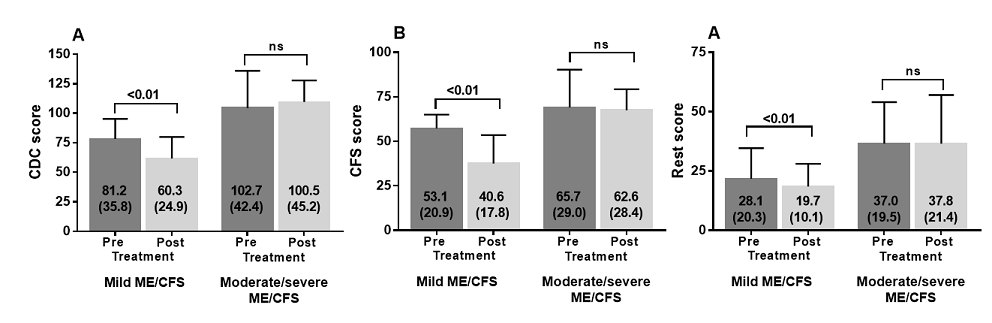
2 shows the pre- and post-treatment values of the three scores in both mild
versus moderate to severe ME/CFS patients. The CDC, CFS, and CDC-other scores
post curcumin use were all significantly lower in the mild ME/CFS group, whereas
not different in the moderate to severe ME/CFS group.In the group with mild
disease (n=26) 13 reported improvement in the global physical and mental
condition, 13 reported no change. In the moderate/severe group, 5 reported
improvement and 20 reported no change (p<0.05). Figure 2:
CDC score pre and post treatment in mild and moderate/severe ME/CFS patients
(panel A), CFS score pre and post treatment in mild and moderate/severe ME/CFS
patients (panel B) and CDC rest score pre and post treatment in mild and
moderate/severe ME/CFS patients (panel C). To the best of our knowledge,
this is the first study that indicates that curcumin use has a positive effect
on symptoms in ME/CFS patients as assessed by the CDC inventory for CFS, and
that those with more severe disease are less likely than those with mild
disease to report improvement. In 2018 we published the first open label
results in ME/CFS patients, showing curcumin might be a
supplement to be prescribed in ME/CFS patients(van Campen 2018). In this larger
group we investigated whether there was a relation between disease severity and
efficacy. Symptoms in ME/CFS include a
perpetual flu-like state, sore throats, tender/swollen lymph nodes, muscle
pain, achy joints without swelling or redness, headaches, chills, feverishness
and sensitivities to foods, odors, and medications, which are all indicative of
inflammation [28].
As increased oxidative stress and altered cytokine patterns have been
demonstrated in ME/CFS, it is likely that curcumin exerts its beneficial
effects by the anti-oxidative
and anti-inflammatory properties.
However, the exact mode of action of curcumin needs to be determined. Moreover,
ME/CFS is considered to be in part a neuro-inflammatory disease. As curcumin
passes the blood-brain barrier it is therefore tempting to assign the positive
effects also to an anti-oxidative and anti-inflammatory effect in the brain
[1-6,31-36,43-49]. Overall, the effect of curcumin
use on ME/CFS symptoms is limited: for the total ME/CFS patient group the total
CDC score decreased from 91.8 (39.9) to 80.0 (40.9), a mean reduction of 12%
(data not shown). The limited efficacy is also obvious from the PROM: change in
physical and mental condition after curcumin use. Thirty-five percent of
patients reported improvement in their physical and mental condition after
curcumin use. The finding that the PROM: improved is associated with a larger
decrease in the total CDC score strengthens the validity of this study. A difference in treatment
efficacy was found between patients with mild disease versus moderate or severe
disease. In the latter group curcumin was less effective. This observation is
not uncommon in other diseases. For example, in a study using a bronchodilator for
COPD (Chronic Obstructive Pulmonary Disease), the medication under study was
less effective in severe COPD vs moderate COPD [50].The reduced efficacy in the
moderate to severe ME/CFS patient may be related to a higher load of
inflammation in this patient group in combination with the low bioavailability
of curcumin. In the unprocessed form the uptake of curcumin is very low. To
improve uptake, encapsulation in liposomes, polymeric nanoparticles,
cyclodextrin encapsulation, lipid complexes, phosphatidylcholine complexes or
polymer-curcumin complexes have been developed, all of which increase the
activity and bioavailability. In a study the absorption of a standardized
curcuminoid mixture and its lecithin formulation was compared [19,51,52]. Total curcuminoid absorption was
about 29-fold higher than for its corresponding unformulated curcuminoid
mixture, but plasma concentrations were still significantly lower than those
required for the inhibition of most anti-inflammatory targets of curcumin.
Remarkably, the phospholipid formulation increased the absorption of
demethoxylated curcuminoid much more than that of curcumin, being a more potent
analogue in many in vitro anti-inflammatory assays. It is possible that an even
higher dose or an increased bioavailability of curcumin and or demethoxylated
curcuminoid may be more effective in moderate to severe ME/CFS patients. These
needs to be studied and further efforts to increase bioavailability and the
clinical effects of the different curcuminoid components are also needed. An interesting finding was that
not only the CFS score decreased significantly in the mild disease group, but
also the CFS-other scores. Some of these symptoms might be related to
inflammation and therefore might respond to curcumin treatment. A larger study
is needed to address the question regarding which specific symptoms are
ameliorated by curcumin. Unexpectedly, a relatively high number of ME/CFS
patients (n=6, 10%) stopped the use of curcumin because of side effects. The
explanation may be that multiple chemical sensitivities are prevalent in ME/CFS
patients. In the study of Brown and Jason, the co-occurrence was almost 24%
[53]. It is unclear whether the drug itself or added excipients cause the
intolerance. The patients completing the study reported no side effects due to
this supplement.The meta-analysis of reported nausea and gastro-intestinal side
effects to be the main reported adverse events. They also reported studies no
showing any side effects. The ME/CFS patients with side effects found these
severe enough to stop the treatment [54]. This study was an open-label
study, without a placebo arm. Some patients (n=8) did not return the first or
second questionnaire, possibly leading to bias. A number of patients in the
outpatient clinic admitted that they had difficulties with completing the
questionnaire. This is most probably due to their memory and concentration
problems, a disabling feature of the disease. A supervised completion approach
may overcome this problem. The meta-analysis of table 1 shows an overview of
trials in which there were different doses and durations of treatment, varying
from single dose interventions up to treatment durations of 22 months [54]. We
chose – empirically in the ME/CFS patient group – for treatment duration of 8
weeks. If a positive response is not present after 8 weeks, the likelihood that
improvement will occur with longer duration is considered to be low. As
patients are not reimbursed for these supplements, we elected to stop treatment
after a few months. Further study would determine whether a longer duration
might increase the percentage of responders. In summary, in this open-labeled
study, 8 weeks of curcumin use in a phosphatidylcholine complex reduced ME/CFS symptomatology,
especially in patients with mild disease, whereas it did not significantly
change symptoms in moderate and severe patients. A randomized
placebo-controlled, larger study is warranted to assess its efficacy in ME/CFS patients. The raw data supporting the
conclusions of this manuscript will be made available by the authors, without
undue reservation, to any qualified researcher. We acknowledge Prof PC Rowe for a
critical review of the paper. 1.
Joe
B and Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in
lowering the generation of reactive oxygen species in rat peritoneal
macrophages (1994) Biochim Biophys Acta 1224: 255-263. https://doi.org/10.1016/0167-4889(94)90198-8 2.
Reddy
A C and B R Lokesh. Effect of dietary turmeric (Curcuma longa) on iron-induced
lipid peroxidation in the rat liver (1994) Food Chem Toxicol 32: 279-283. https://doi.org/10.1016/0278-6915(94)90201-1 3.
Kang
J, Chen J, Shi Y, Jia J and Zhang Y. Curcumin-induced histone hypoacetylation:
the role of reactive oxygen species (2005) Biochem Pharmacol 69: 1205-1213. https://doi.org/10.1016/j.bcp.2005.01.014 4.
Jeong
GS, Oh GS, Pae HO, Jeong SO, Kim YC et al. Comparative effects of curcuminoids
on endothelial heme oxygenase-1 expression: ortho-methoxy groups are essential
to enhance heme oxygenase activity and protection (2006) Exp Mol Med 38:
393-400. https://doi.org/10.1038/emm.2006.46 5.
Abdel-Daim
MM and Abdou RH. Protective Effects of Diallyl Sulfide and Curcumin Separately
against Thallium-Induced Toxicity in Rats (2015) Cell J 17: 379-388. 6.
Joe
B, Vijaykumar M and Lokesh B R. Biological properties of curcumin-cellular and
molecular mechanisms of action (2004) Crit Rev Food Sci Nutr 44: 97-111. 7. Wright
LE, Frye JB, Gorti B, Timmermann BN and Funk JL. Bioactivity of
turmeric-derived curcuminoids and related metabolites in breast cancer (2013)
Curr Pharm Des 19: 6218-6225. https://doi.org/10.2174/1381612811319340013 8.
Mahady
GB, Pendland SL, Yun G and Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit
the growth of Helicobacter pylori, a group 1 carcinogen (2002) Anticancer Res
22: 4179-4181. 9.
Reddy
RC, Vatsala PG, Keshamouni VG, Padmanaban G and Rangarajan PN. Curcumin for
malaria therapy (2005) Biochem Biophys Res Commun 326: 472-474. https://doi.org/10.1016/j.bbrc.2004.11.051 10.
Sethi
G, Sung B and Aggarwal BB. Nuclear factor-kappaB activation: from bench to
bedside (2008) Exp Biol Med (Maywood) 233: 21-31. https://doi.org/10.3181/0707-mr-196 11. Reuter
S, Gupta SC, Chaturvedi MM and Aggarwal BB. Oxidative stress, inflammation, and
cancer: how are they linked? (2010) Free Radic Biol Med 49: 1603-1616. https://doi.org/10.1016/j.freeradbiomed.2010.09.006 12.
Cho
JW, Lee KS and Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6,
and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB
and MAPKs as potential upstream targets (2007) Int J Mol Med 19: 469-474. https://doi.org/10.3892/ijmm.19.3.469 13.
Huang
CZ, Huang WZ, Zhang G and Tang DL. In vivo study on the effects of curcumin on
the expression profiles of anti-tumour genes (VEGF, CyclinD1 and CDK4) in liver
of rats injected with DEN (2013) Mol Biol Rep 40: 5825-5831. https://doi.org/10.1007/s11033-013-2688-y 14. Li
R, Wang Y, Liu Y, Chen Q, Fu W, et al. Curcumin inhibits transforming growth
factor-beta1-induced EMT via PPARgamma pathway, not Smad pathway in renal
tubular epithelial cells (2013) PLoS One 8. https://doi.org/10.1371/journal.pone.0058848 15.
Anthwal
A, Thakur BK, Rawat MS, Rawat DS, Tyagi AK, et al. Synthesis, characterization
and in vitro anticancer activity of C-5 curcumin analogues with potential to
inhibit TNF-alpha-induced NF-kappaB activation (2014) Biomed Res Int 1–10. https://doi.org/10.1155/2014/524161 16. Nieto
CI, Cabildo MP, Cornago MP, Sanz D, Claramunt RM, et al. Fluorination Effects
on NOS Inhibitory Activity of Pyrazoles Related to Curcumin (2015) Molecules
20: 15643-15665. https://doi.org/10.3390/molecules200915643 17.
Paulino
N, Paulino AS, Diniz SN, de Mendonca S, Goncalves ID, et al. Evaluation of the
anti-inflammatory action of curcumin analog (DM1): Effect on iNOS and COX-2
gene expression and autophagy pathways (2016) Bioorg Med Chem 24: 1927-1935. https://doi.org/10.1016/j.bmc.2016.03.024 18.
de
Porras RV, Bystrup S, Martinez-Cardus A, Pluvinet R, Sumoy L, et al. Curcumin
mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell
lines through modulation of CXC-Chemokine/NF-kappaB signalling pathway (2016)
Sci Rep 6. https://doi.org/10.1038/srep24675 19.
Pulido-Moran
M, Moreno-Fernandez J, Ramirez-Tortosa C and Ramirez-Tortosa C Curcumin and
Health (2016) Molecules 21: 264. https://doi.org/10.3390/molecules21030264 20.
Pari
L, Tewas D and Eckel J. Role of curcumin in health and disease (2008) Arch
Physiol Biochem 114: 127-149. 21.
Akazawa
N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R and Maeda S. Curcumin
ingestion and exercise training improve vascular endothelial function in
postmenopausal women (2012) Nutr Res 32: 795-799. https://doi.org/10.1016/j.nutres.2012.09.002 22. Panahi
Y, Rahimnia AR, Sharafi M, Alishiri G,Saburi A et al. Curcuminoid treatment for
knee osteoarthritis: a randomized double-blind placebo-controlled trial (2014)
Phytother Res 28: 1625-1631. https://doi.org/10.1002/ptr.5174 23. Ahn
JK, Kim S, Hwang J, Kim J, Lee YS et al. Metabolomic Elucidation of the Effects
of Curcumin on Fibroblast-Like Synoviocytes in Rheumatoid Arthritis (2015) PLoS
One 10. https://doi.org/10.1371/journal.pone.0145539 24.
Lang
A, Salomon N, Wu JCY, Kopylov U, Lahat A et al. Curcumin in combination with
mesalamine induces remission in patients with mild-to-moderate ulcerative
colitis in a randomized controlled trial (2015) Clin Gastroenterol Hepatol 13:
1444-1449. https://doi.org/10.1016/j.cgh.2015.02.019 25.
Yu
JJ, Pei LB, Zhang Y, Wen ZY and Yang JL. Chronic supplementation of curcumin
enhances the efficacy of antidepressants in major depressive disorder: a
randomized, double-blind, placebo-controlled pilot study (2015) J Clin
Psychopharmacol 35: 406-410. https://doi.org/10.1097/jcp.0000000000000352 26.
Rainey-Smith
SR, Brown BM, Sohrabi HR, Shah T and Goozee KG. et al. Curcumin and cognition:
a randomised, placebo-controlled, double-blind study of community-dwelling
older adults (2016) Br J Nutr 115: 2106-2113. https://doi.org/10.1017/S0007114516001203 27.
Tenero
L, Piazza M, Zanoni L, Bodini A, Peroni D, et al. Antioxidant supplementation
and exhaled nitric oxide in children with asthma (2016) Allergy Asthma Proc 37:
8-13. https://doi.org/10.2500/aap.2016.37.3920 28.
Institute
of Medicine. Beyond mayalgic encephalomyelitis/chronic fatigue syndrome:
redefining an illness (2015) The National Academies Press, Washington DC,
United States. 29.
Jason
LA and Richman JA. How science can stigmatize: The case of chronic fatigue
syndrome (2008) J Chronic Fatigue Syndrome 53: 1195–1219. https://doi.org/10.3109/10573320802092146 30.
Twisk
FN. The status of and future research into Myalgic Encephalomyelitis and
Chronic Fatigue Syndrome: the need of accurate diagnosis, objective assessment
and acknowledging biological and clinical subgroups (2014) Front Physiol 5:
109. https://doi.org/10.3389/fphys.2014.00109
31.
Jason
L, Sorenson M, Sebally K, Alkazemi D, Lerch A, et al. Increased HDAC in
association with decreased plasma cortisol in older adults with chronic fatigue
syndrome. (2011) Brain Behav Immun. 32.
Morris
G, Anderson G, Dean O, Berk M and Galecki P et al. The glutathione system: a
new drug target in neuroimmune disorders (2014) Mol Neurobiol 50: 1059-1084. 33.
Nijs
J, Nees A, Paul L, De Kooning M, Ickmans K. et al. Altered immune response to
exercise in patients with chronic fatigue syndrome/myalgic encephalomyelitis: a
systematic literature review. Exerc Immunol Rev 20: 94-116. 34.
Maes,
Bosmans M E and Kubera M. Increased expression of activation antigens on CD8+ T
lymphocytes in Myalgic Encephalomyelitis/chronic fatigue syndrome: inverse
associations with lowered CD19+ expression and CD4+/CD8+ ratio, but no
associations with (auto)immune, leaky gut, oxidative and nitrosative stress
biomarkers (2015) Neuro Endocrinol Lett 36: 439-446. 35.
Morris
G, Berk M, Walder K and Maes M. Central pathways causing fatigue in neuro
inflammatory and autoimmune illnesses (2015) BMC Med 13: 28. https://doi.org/10.1186/s12916-014-0259-2 36.
Blundell
S, Ray KK, Buckland M and White PD. Chronic fatigue syndrome and circulating
cytokines: A systematic review (2015) Brain Behav Immun 50: 186-195. https://doi.org/10.1016/j.bbi.2015.07.004 37 van
Campen CLMCRKV, F.C. The effect of Curcumin on patients with Chronic Fatigue
Syndrome/Myalgic Encephalomyelitis: an open label study (2018) Int J Clinical
Medicine 9: 356-366. https://doi.org/10.4236/ijcm.2018.95031 38.
Fukuda
K, Straus SE, Hickie I, Sharpe MC, Dobbins JG et al. The chronic fatigue syndrome:
a comprehensive approach to its definition and study. International Chronic
Fatigue Syndrome Study Group (1994) Ann Intern Med 121: 953-959. https://doi.org/10.7326/0003-4819-121-12-199412150-00009 39. Carruthers
BM, van de Sande MI, DE Meirleir KL, Klimas NG, Broderick G, et al. Myalgic
encephalomyelitis: International Consensus Criteria (2018) J Intern Med 270:
327-338. https://doi.org/10.3390/diagnostics9010001 40. Wagner
DR, Nisenbaum C, Heim JF, Jones ER, Unger et al. Psychometric properties of the
CDC symptom inventory for assessment of chronic fatigue syndrome (2005) Popul
Health Metr 3: 8. http://doi.org/10.1186/1478-7954-3-8 41.
Vermeulen
RC. Translation and validation of the dutch language version of the CDC symptom
inventory for assessment of Chronic Fatigue Syndrome (CFS) (2006) Popul Health
Metr 4: 12. http://doi.org/10.1186/1478-7954-4-12 42.
Viehoff
PB, van Genderen FR and Wittink H. Upper limb lymphedema 27 (ULL27): Dutch
translation and validation of an illness-specific health-related quality of
life questionnaire for patients with upper limb lymphedema (2008) Lymphology
41: 131-138. 43.
Hornig
M, Montoya JG, Klimas NG, Levine S, Felsenstein D, et al. Distinct plasma
immune signatures in ME/CFS are present early in the course of illness (2015)
Sci Adv 1. http://doi.org/10.1126/sciadv.1400121 44.
Hornig
M, Gottschalk G, Peterson DL, Knox KK, Schultzm AF, et al. Cytokine network
analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue
syndrome (2016) Mol Psychiatry 21: 261-269. http://doi.org/10.1038/mp.2015.29 45. Landi
AD, Broadhurst SD, Vernon DL, Tyrrell et al. Reductions in circulating levels
of IL-16, IL-7 and VEGF-A in myalgic encephalomyelitis/chronic fatigue syndrome
(2016) Cytokine 78: 27-36. http://doi.org/10.1016/j.cyto.2015.11.018 46.
Russell
L,Broderick G, Taylor R, Fernandes H, Harvey J, et al. Illness progression in
chronic fatigue syndrome: a shifting immune baseline (2016)BMC Immunol 17: 3. http://doi.org/10.1186/s12865-016-0142-3 47.
Nakatomi
Y, Mizuno K, Ishii A, Wada Y, Tanaka M, et al. Neuroinflammation in Patients
with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An 11C-(R)-PK11195 PET
Study (2014) J Nucl Med 55: 945-950. http://dx.doi.org/10.2967/jnumed.113.131045 48.
Morris,
G., M. Berk, P. Galecki, K. Walder and M. Maes. The neuro-immune
pathophysiology of central and peripheral fatigue in systemic
immune-inflammatory and neuro-immune diseases (2016) Mol Neurobiol 53:
1195-1219. http://doi.org/10.1007/s12035-015-9090-9 49.
Ryu
EK, Choe YS, Lee KH, Choi Y and Kim BT. Curcumin and dehydrozingerone
derivatives: Synthesis, radiolabeling, and evaluation for beta-amyloid plaque
imaging (2006) J Med Chem 49: 6111-6119. http://doi.org/10.1021/jm0607193 50.
Chapman
KR, Bateman ED, Chen H, Hu H, Fogel R, et al. QVA149 improves lung function,
dyspnea, and health status independent of previously prescribed medications and
copd severity: a subgroup analysis from the SHINE and ILLUMINATE studies (2015)
Chronic Obstr Pulm Dis 2: 48-60. http://doi.org/10.15326/jcopdf.2.1.2014.0140 51.
Prasad
S, Tyagi AK and Aggarwal BB. Recent developments in delivery, bioavailability,
absorption and metabolism of curcumin: The golden pigment from golden spice (2014)
Cancer Res Treat 46: 2-18. http://doi.org/10.4143/crt.2014.46.1.2 52. Cuomo
J, Appendino G, Dern AS, Schneider E, McKinnon TP, et al. Comparative
absorption of a standardized curcuminoid mixture and its lecithin formulation
(2011) J Nat Prod 74: 664-669. http://doi.org/10.1021/np1007262 53. Brown
MM and Jason LA. Functioning in individuals with chronic fatigue syndrome:
increased impairment with co-occurring multiple chemical sensitivity and
fibromyalgia (2007) Dyn Med 6: 9. http://doi.org/10.1186/1476-5918-6-6 Gupta SC, Patchva S and Aggarwal BB. Therapeutic
roles of curcumin: lessons learned from clinical trials (2013) AAPS J 15:
195-218. http://doi.org/10.1208/s12248-012-9432-8 54.
Gupta
SC, Patchva S and Aggarwal BB. Therapeutic roles of curcumin: lessons learned
from clinical trials (2013) AAPS J 15: 195-218. http://doi.org/10.1208/s12248-012-9432-8 *Corresponding
author C
(Linda) MC van Campen, Department of Cardiology, Stichting Cardiozorg,
Hoofddorp, Netherlands, E-mail: info@stichtingcardiozorg.nl Citation Campen
CMCV and Visser FC. The effect of curcumin in patients with chronic fatigue
syndrome/myalgic encephalomyelitis: disparate responses in different disease
severities (2019) Pharmacovigil and Pharmacoepi 2: 22-27. Curcumin, Myalgic Encephalomyelitis/Chronic
Fatigue Syndrome, Disease severity, CDC score, Fukuda score, R and 36
questionnaire.The Effect of Curcumin in Patients with Chronic Fatigue Syndrome (or) Myalgic Encephalomyelitis Disparate Responses in Different Disease Severities
C(Linda) MC van
Campen*and Frans C Visser
Abstract
Full-Text
Introduction
Patients
and Methods
Data
analysis
Statistical
Analysis
Results
Discussion
Limitations
Conclusion
Data
Availability Statement
Acknowledgments
References
Keywords