Review Article :
Ilia Davarashvili and Jonathan
Balkin Objectives: The Guillain-Barre Syndrome (GBS) is a cause of
acute flaccid paralysis mainly in young and middle-aged adults and commonly
requires admission to an intensive care unit. Manifestations of the GBS vary
from monoparesis to life-threatening progressive ascending paralysis with the
involvement of the respiratory muscles. The latter often accompanied with
cardiac involvement. There
is a wide range of clinical cardiac manifestations: from signs of autonomic
dysfunction (labile blood pressure, oscillations in heart rate) to involvement
of the myocardium and potentially fatal arrhythmias. Materials and methods: We present a case of a patient with GBS complicated
with ventricular tachycardia. The accompanying review of the literature
underlines the wide spectrum of cardiac complications in this entity. Results and Conclusions: A thorough review of the literature shows rare
reports of a wide spectrum of cardiac abnormalities, with no reported
spontaneous VT. We suggest that careful cardiac assessment of patients with GBS
be performed including continuous ECG monitoring as well as measurement of
cardiac enzymes and 2-D Echocardiography. A 71-year-old male with
hyperlipidemia, hypertension, Paroxysmal Atrial Fibrillation (PAF), Ischemic
Heart Disease (IHD), Ischemic Cerebrovascular Accident (CVA), with permanent
ICD for primary prevention was admitted to the neurological department with a paresthesia
and numbness of the face and extremities. On neurological examination extreme
proximal muscle weakness with poor sensation of palms and soles, depressed
reflexes and unsteady walking. The cranial CT showed old small infarcts. His
background treatment included: P.O. Aspirin 100 mg q.d., P.O. Atorvastatin 40
mg q.d., P.O. Enaladex 5 mg q.d., P.O. Sotalol 40 mg b.i.d. He was started on
treatment with plasmapheresis and 3 days later IVIG treatment was added. During
admission bacteremia with Klebsiella Pneumonia on blood culture and treatment
with Tazocin was started. Three days later the patient
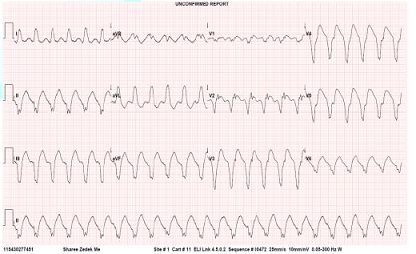
began to have recurrent episodes of sustained VT (Figure 1). The ICD did not sense the events because the threshold
rate for VT detection was higher than the actual rate of VT in this patient.
The patient was hemodynamically stable during each episode of VT. Treatment was
started with Amiodarone, beta-blockers and the patient received DCA cardio
version on two occasions. Figure 1: ECG showing Ventricular Tachycardia. Intravenous Lidocaine was begun
and patient was transferred to the ICCU. Laboratory examination: Electrolytes, creatinine
was WNL. WBC was 17.2 K/uL (normal range 5-10 K/ul) with Neutrophil count
predominance, CRP was 11.4 mg/dL (normal range 0-0.5 mg/dL). Cardiac enzymes
normal. Prothrombin time 12.9 s (INR of 1.07), and a partial thromboplastin
time normal. While treated with amiodarone and lidocaine no further
tachyarrhythmias detected. The Guillain-Barre syndrome,
which is characterized by acute areflexic paralysis with albuminocytologic
dissociation (i.e., high levels of protein in the cerebrospinal fluid and
normal cell counts), was described in 1916 [1]. Since poliomyelitis has been
eliminated, the Guillain-Barre syndrome is currently the most frequent cause of
acute flaccid paralysis worldwide and constitutes one of the serious
emergencies in neurology [2].
The presentation of GBS may be acute
or sub-acute in presentation [2]. It affects the peripheral nerves and is
characterized by symmetrical progressive ascending weakness with areflexia and
variable sensory complaints [3,4]. The GBS is presumed to be caused by an
aberrant auto immune response against peripheral nerves by cross-reacting
antibodies [5,6]. The incidence of the GBS is estimated at 1 to 2 per 100,000
per year with a preponderance in women over 50 years of age [7,8]. GBS is often
preceded by an infection that is believed to evoke an immune response [9]. The
classification is based on nerve-conduction studies and there is a notable
difference in the geographic distribution of subtypes of the syndrome [10,11]
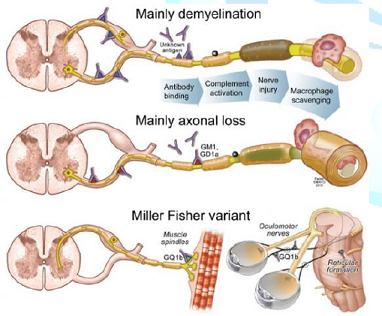
The classic pathological findings in acute inflammatory demyelinating
polyneuropathy are inflammatory infiltrates (consisting mainly of T cells and
macrophages) and areas of segmental demyelination, often associated with signs
of secondary axonal degeneration, which can be detected in the spinal roots, as
well as in the large and small motor and sensory nerves (Figure 2) [12]. The immune response leads to a cross-reaction with
peripheral nerve components because of shared epitopes resulting in acute
polyneuropathy [13]. This is further supported by the identification of various
antiganglioside antibodies noted in necropsy and animal models that cross-react
with the ganglioside surface molecules of peripheral nerves [9,12]. This
phenomenon may also explain the potential involvement of the heart, which
possesses lactose-containing gangliosides. Figure 2: Guillain-Barré syndrome pathogenesis.
Different degrees of affliction
of the autonomic nervous system can be seen in up to 70% of patients with the
GBS [13]. Current data suggest sympathetic over activity rather than
parasympathetic hypo activity in such patients [14]. It is postulated that a
failure of catecholamine uptake in the “irritated” peripheral nerves may be responsible
for this activity [15]. In addition, the denervated organs have been noted to
be increasingly sensitive to catecholamines, resulting in denervation
hypersensitivity [16].
Cardiovascular disturbances are
believed to be secondary to a combination of this entity in addition to impairment
of the carotid sinus reflex [17]. Cardiovascular abnormalities in the GBS have
been attributed to autonomic
neuropathy in the efferent fibers of the vagus nerves, and are seen in about of
70% of affected patients [18]. However,
autopsy findings have not confirmed these changes [19,20]. Other cardiac complications have
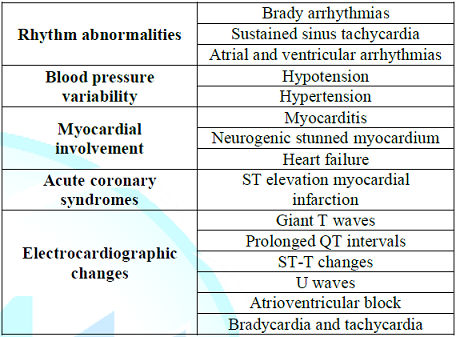
also been described in the patients with Guillian-Barre syndrome, mainly: heart
rate variability, BP variability, cardiomyopathy, and electrocardiographic
changes (Table 1) [12,21,22]. Table 1: Common cardiovascular complications of the Guillain-Barré syndrome. Sustained sinus tachycardia is
the most common abnormality. This was believed to be due to sympathetic
hyperactivity [14]. Due to its transient
nature, the treatment is usually supportive [23]. Other tachyarrhythmias,
including atrial and ventricular arrhythmias, may also occur [22]. There have
been two anecdotal reports of ventricular tachycardia and fibrillation after
administration of muscle relaxants for tracheal intubation or tracheostomy [24].
There have been no other reports in the literature of spontaneous VT in
patients with GBS. We know that there is a wide spectrum of main etiologies of
VT like cardiomyopathies (with coronary disease), long QT and Brugada syndrome
as well as adverse effects of different medications and electrolyte imbalances
[25]. Our patient had known CAD with severe left ventricular dysfunction and
this underlying condition may have responsible for the VT occurring during this
acute illness. Bradyarrhythmias including
atrioventricular block and asystole, have been reported in 7% to 34% of
patients and may occur in up to 50% of patients with GBS, and are potentially
serious events necessitating the administration of atropine or pacemaker
placement [14,26]. Vagal over activity caused by afferent baroreceptors reflex
failure is believed to be a pathogenesis for bradycardia. Aggressive correction
of associated factors such as hypoxia, medication side effects, and metabolic
abnormalities may help in prevention [27]. Where severe bradycardia has been
described no published consensus has been reached on whether to implant a temporary
or permanent pacemaker-as recovery of these patients and infective complications
are hard to predict [28]. Blood Pressure (BP) variability
can be attributed to disturbances in the baroreceptor reflex pathway as well as
to changes in the catecholamine levels. The dysregulation of the
parasympathetic and sympathetic systems is responsible for alterations in
venomotor tone and peripheral vascular resistance, most often causing
transient, but in some cases, persistent hypotension. Fluctuations in BP are
often considered as pathognomonic for the GBS and are likely to occur in critical
illnesses or neuropathy [29]. Although these episodes of BP
deviation were most often related to mechanical ventilation, analgesia, and
sedation they may also occur without sedation [14]. There are no specific
recommendations for target mean arterial pressure and the treatment is mainly
symptomatic either with fluids and inotropes for hypotension and with IV
antihypertensive therapy and/or vasodepressors for hypertension. The possible
mechanisms are denervation hypersensitivity, but other contributing conditions,
such as pulmonary thromboembolism, hypoxemia, sepsis, gastrointestinal
bleeding, and metabolic abnormalities, need to be considered [13,30]. Myocardial involvement ranges
from asymptomatic myocarditis to neurogenic stunned myocardium and heart
failure. It can arise from the activation of the sympathetic nervous system, caused
by catecholamine-associated myocardial injury but infectious, chemicals and
hypersensitivity medications can also account for this damage [31,32]. It is
possible that the extent of myocardial involvement has been underestimated as
routine 2-dimensional echocardiography is not performed in this critically ill
cohort of patients (including on mechanical ventilation) [33]. There are anecdotal reports of
acute coronary syndromes, including ST-segment elevation myocardial infarction
occurring during therapy for GBS with intravenous immunoglobulin [34]. In
another report intracoronary Doppler flow measurements revealed an elevated
baseline coronary flow velocity with a decreased coronary flow reserve,
supposedly secondary to a catecholamine surge [33]. A wide spectrum of
electrocardiographic changes have been demonstrated, including giant T waves,
prolonged QT intervals, ST-T changes, U waves, and atrioventricular block, in
addition to bradycardia and tachycardia as previously described [35]. These
changes are also believed to be secondary to associated myocardial involvement.
Along with 2-dimensional echocardiographic studies, other modalities to
demonstrate cardiac involvement such as iodine-123 meta-iodobenzylguanidine
myocardial scintigraphy and carbon-11 hydroxyephedrine positron emission
tomography can also be used to study sympathetic innervation of myocardium [36].
GBS is the most common cause of
acute flaccid paralysis in young adults and the elderly and an important cause
of admission to intensive care units. Critically ill patients with paralysis
and need for mechanical ventilation often have cardiac involvement. This ranges
from variations in blood pressure to involvement of the myocardium and potentially
fatal arrhythmias. A thorough review of the literature shows rare reports of a
wide spectrum of cardiac abnormalities, with no reported spontaneous VT. We
suggest that careful cardiac assessment of patients with GBS be performed
including continuous ECG monitoring as well as measurement of cardiac enzymes
and 2-D Echocardiography.
1. Guillain
G, Barré JA and Strohl A. Sur un syndrome de radiculonévrite avec
hyperalbuminose du liquide céphalo-rachidien sans réaction cellulaire:
remarques sur les caractères cliniques et graphiques des reflexes tendineux
(1916) Bulletins et mémoires de la Société des Médecins des Hôpitaux de Paris
40: 1462-1470. 2. Nobuhiro
Y and Hartung H. Guillain–Barré Syndrome (2012) NEJM 366: 2294-2304. https://doi.org/10.1056/NEJMra1114525 3. Koeppen
S, Kraywinkel K, Wessendorf TE, Ehrenfeld CE, Schürks M, et al. Long-term
outcome of Guillain-Barré syndrome (2006) Neurocrit Care 5: 235-242. https://doi.org/10.1385/NCC:5:3:235 4. Teitelbaum
JS and Borel CO. Respiratory dysfunction in Guillain-Barré syndrome (1994) Clin
Chest Med 15: 705-714. 5. Flachenecker
P. Autonomic dysfunction in Guillain-Barré syndrome and multiple sclerosis (2007)
J Neurol 254: 96-101. https://doi.org/10.1007/s00415-007-2024-3 6. Hartung
HP, Pollard JD, Harvey GK and Toyka KV. Immunopathogenesis and treatment of the
Guillain-Barré syndrome-part I (1995) Muscle Nerve 18: 137-153. https://doi.org/10.1002/mus.880180202 7. McGrogan
A, Madle GC, Seaman HE and de Vries CS. The epidemiology of Guillain-Barré
syndrome worldwide. A systematic literature review (2008) Neuroepidemiology 32:
150-163. https://doi.org/10.1159/000184748 8. Jones
HR Jr. Guillain-Barré syndrome: perspectives with infants and children (2000)
Semin Pediatr Neurol 7: 91-102. https://doi.org/10.1053/pb.2000.6690 9. Hahn
AF. Guillain-Barré syndrome (1998) Lancet 352: 635-641. https://doi.org/10.1016/S0140-6736(97)12308-X 10. Hadden
RD, Cornblath DR, Hughes RA, Zielasek J, Swan AV, et al. Electrophysiological
classification of Guillain-Barré syndrome: clinical associations and outcome
(1998) Ann Neurol 44: 780-788. https://doi.org/10.1002/ana.410440512 11. Ho
TW, Mishu B, Li CY, Gao CY, Blaser MJ, et al. Guillain-Barré syndrome in
northern China: relationship to Campylobacter Jejuni infection and
anti-glycolipid antibodies (1995) Brain 118: 597-605. https://doi.org/10.1093/brain/118.3.597 12. Finkelstein
JS and Melek BH. Guillain-Barré syndrome as a cause of reversible
cardiomyopathy (2006) Tex Heart Inst J 33: 57-59. 13. Zochodne
DW. Autonomic involvement in Guillain-Barré syndrome: a review (1994) Muscle
Nerve 17: 1145-1155. https://doi.org/10.1007/s10286-018-0542-y 14. Pfeiffer
G, Schiller H, Kruse J and Netzer J. Indicators of dysautonomia in severe
Guillain-Barré syndrome (1999) J Neurol 246: 1015-1022. https://doi.org/10.1007/s004150050506 15. Ahmad
J, Kham AS and Siddiqui MA. Estimation of plasma and urinary catecholamines in
Guillain-Barré syndrome (1985) Japanese J Med 24: 24-29. https://doi.org/10.2169/internalmedicine1962.24.24 16. Asahina
M, Kuwabara S, Suzuki A and Hattori T. Autonomic function in demyelinating and
axonal subtypes of Guillain-Barré syndrome (2002) Acta Neurol Scand 105: 44-50.
https://doi.org/10.1034/j.1600-0404.2002.00099.x 17. Mitchell
PL and Meilman E. The mechanism of hypertension in the Guillain-Barré syndrome
(1967) Am J Med 42: 986-995. https://doi.org/10.1016/0002-9343(67)90079-4 18. Flachenecker
P, Wermuth P, Hartung HP and Reiners K. Quantitative assessment of
cardiovascular autonomic function in Guillain-Barré syndrome (1997) Ann Neurol
42: 171-179. https://doi.org/10.1002/ana.410420207 19. Tuck
RR and McLeod JG. Autonomic dysfunction in Guillain-Barré syndrome (1981) J
Neurol Neurosurg Psychiatry 44: 983-990. http://dx.doi.org/10.1136/jnnp.44.11.983 20. Bredin
CP. Guillain-Barré syndrome: the unsolved cardiovascular problems (1977) Ir J
Med Sci 146: 273-279. https://doi.org/10.1007/BF03030974 21. Annane
D, Baudrie V, Blanc AS, Laude D, Raphaël JC, et al. Short-term variability of
blood pressure and heart rate in Guillain-Barré syndrome without respiratory
failure (1999) Clin Sci 96: 613-621. https://doi.org/10.1042/cs0960613 22. Mukerji
S, Aloka F, Farooq M, Kassab M and Abela G. Cardiovascular Complications of the
Guillain-Barré Syndrome (2009) Am J Cardiol 104: 1452-1455. https://doi.org/10.1016/j.amjcard.2009.06.069 23. Flachenecker
P, Hartung HP and Reiners K. Power spectrum analysis of heart rate variability
in Guillain-Barré syndrome. A longitudinal study (1997) Brain 120: 885-894.
https://doi.org/10.1093/brain%2F120.10.1885 24. Graham
IF. The heart in the Guillian-Barre syndrome (1984) British Medical J 288:
6411. 25. Tonelli
A, Khasnis A and Abela GS. Peaked T-waves and sinus arrhythmia before prolonged
sinus pauses and atrioventricular block in the Guillain-Barré Syndrome (2007)
Indian Pacing Electrophysiol J 7: 249-252. 26. Kordouni
M, Jibrini M and Siddiqui MA. Long-term transvenous temporary pacing with
active fixation bipolar lead in the management of severe autonomic dysfunction
in Miller-Fisher syndrome: a case report (2007) Int J Cardiol 117: 10-12.
https://doi.org/10.1016/j.ijcard.2006.07.086 27. Hund
EF, Borel CO, Cornblath DR, Hanley DF and McKhann GM. Intensive management and
treatment of severe Guillain-Barré syndrome
(1993) Crit Care Med 21: 433-436. 28. Wijdicks
EF, Litchy WJ, Harrison BA and Gracey DR. The clinical spectrum of critical
illness polyneuropathy (1994) Mayo Clin Proc 69: 955-959. http://dx.doi.org/10.1016/S0025-6196(12)61819-9 29. Ropper
AH. Critical care of Guillain-Barré syndrome. In: (Ed) Ropper AH (2003)
Neurological and neurosurgical intensive care. 4th ed. Philadelphia:
Lippincott, Williams & Wilkins 278-298. 30. Aslam
AF, Aslam AK, Vasavada BC and Khan IA. Cardiac effects of acute myelitis (2006)
Int J Cardiol 111: 166 -168. https://doi.org/10.1016/j.ijcard.2005.06.018 31.Goldman
MJ and Makaryus AN. Guillain-Barré syndrome complicated by myocarditis (2006)
Mt Sinai J Med 73: 1126 -1128. 32. Barsheshet
A, Marai I, Appel S and Zimlichman E. Acute ST elevation myocardial infarction
during intravenous immunoglobulin infusion (2007) Ann N Y Acad Sci 1110:
315-318. https://doi.org/10.1196/annals.1423.033 33. Yoshii
F, Kozuma R, Haida M, Shinohara Y, Yoshitake M, et al. Giant negative T waves
in Guillain-Barré syndrome (2000) Acta Neurol Scand 101: 212-215. https://doi.org/10.1034/j.1600-0404.2000.101003212.x 34. Dagres
N, Haude M, Baumgart D, Sack S and Erbel R. Assessment of coronary morphology
and flow in a patient with Guillain-Barré syndrome and ST-segment elevation
(2001) Clin Cardiol 24: 260-263. https://doi.org/10.1002/clc.4960240318 35. Münch
G, Nguyen NT, Nekolla S, Ziegler S, Muzik O, et al. Evaluation of sympathetic
nerve terminals with [(11)C] epinephrine and [(11)C] hydroxyephedrine and
positron emission tomography (2000) Circulation 101: 516-523. https://doi.org/10.1161/01.CIR.101.5.516 36. Priori S. 2015 ESC Guidelines for the management
of patients with ventricular arrhythmias and the prevention of sudden cardiac
death (2015) European Heart J 36: 2793-2867. https://doi.org/10.1093/eurheartj/ehv316 Ilia Davarashvili, Jesselson Integrated Heart Center, Shaare Zedek Medical Center, the Hebrew University of Jerusalem, Jerusalem, Israel, Tel:972-2-6555320, E-mail: idavarashvili@yahoo.com Davarashvili I and Balkin J.Ventricular tachycardia in the guillain-barre syndrome. Cardiac complications in guillain-barre syndrome, review of the literature (2018) Clinical Cardiol Cardiovascular Med 2: 8-11.Ventricular Tachycardia in the Guillain-Barre Syndrome. Cardiac Complications in Guillain-Barre Syndrome, Review of the Literature
Abstract
Full-Text
Case
presentation
Discussion
Conclusions
References
*Corresponding author:
Citation: