Case Report :
We
report the case of an 18 year old man who unexpectedly died of healing
myocarditis. His heart was hypertrophied with multifocal fibrosis which can be
a common histological feature of primary and secondary cardiomyopathy as well
as the healing phase of myocarditis. However, the pattern of myocardial fibrosis,
inflammation with myonecrosis, sparing of the right ventricular myocardium, and
cardiomyocytes features in the remaining areas of the heart were considered as
the key elements in determining a diagnosis of myocarditis. This case
illustrates that meticulous histologic examination and the analysis of the
histologic findings in the hypertrophied heart with multifocal fibrosis can be
helpful to make a correct diagnosis. Sudden cardiac death is one of the most common causes of
death in the practice of forensic pathology. It refers to an unexpected and
sudden death where sudden cessation of cardiac activity occurred with the
hemodynamic collapse due to ventricular tachycardia, or ventricular
fibrillation [1]. This ventricular arrhythmia usually occurs in the setting of
an underlying myocardial disease, which can lead to uneven or a disorganized
depolarization and repolarization [2,3]. Sudden cardiac death in young adults is an important issue
for public health and safety in order to prevent premature death. Myocarditis
and cardiomyopathy including hypertrophic cardiomyopathy and arrhythmogenic
ventricular cardiomyopathy are the most common underlying myocardial diseases
resulting in sudden deaths in this group [4]. Since myocarditis is ongoing into
the healed or healing myocarditis; it is difficult to distinguish it from primary
cardiomyopathy. Although, in most cases of myocarditis, the role for
vaccination and infection control in preventing myocarditis is unknown. However, vaccination may be effective in preventing some of
viral myocarditis [5]. As it can be genetic, it is also important to confirm
the specific type of primary cardiomyopathy for the deceaseds family and
recommend further management. Therefore, we presented a case of a sudden death
in a young man with a hypertrophied heart and multifocal fibrosis, which was
determined to be healing myocarditis that was distinguished from primary
cardiomyopathy. The deceased was an 18-year-old male who was found dead
unexpectedly in his bed by his father. When the paramedics arrived clear signs
of death and postmortem changes such as livor mortis and rigor mortis were
observed. The deceased was healthy, without a significant past medical history
and was not known to use illicit drugs or consume significant quantities of
alcohol. On external examination, lividity was present on the front of the
body. There were no injuries or needle marks. On internal examination, the
heart appeared enlarged, globular and somewhat flabby, and sectioning revealed
multifocal fibrosis. No blood or effusions were identified within the
unremarkable pericardium. There was no evidence of significant atherosclerotic
luminal stenosis on the coronary arteries. There were no significant findings
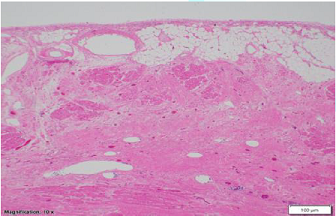
on any other internal organs. The microscopic examination showed subacute lymphocytic myocarditis
with evidence of early secondary cardiomyopathic changes; moderate to marked
interstitial and replacement-type fibrous tissue deposition in a band-like
distribution within the mid myocardial and subepicardial third in the left
ventricular wall and the interventricular septum. Moderate to marked
lymphocytic infiltrates associated with myonecrosis and cardiomyocyte dropout
in the interventricular septum as well as scattered and minute lymphocytic
infiltrates in the free wall. Only a focal and minimal interstitial fibrous
tissue deposition was noted in the right ventricular myocardium. There were no
significant pathological findings in the other organs (Figure 1). A nasopharyngeal swab was performed for viral studies and
Respiratory Syncytial Virus B was detected by nucleic acid amplification test.
Toxicological testing showed negative result for drugs and alcohol. Genetic
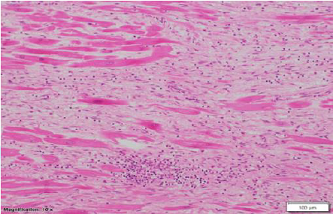
testing was performed and was negative for the cardiomyopathy panel. This case describes the key differential histological
findings for a diagnosis of healing myocarditis in a hypertrophied heart with
multifocal fibrosis, distinguished from cardiomyopathy. The main macroscopic
findings at autopsy showed hypertrophied heart (enlargement of right atrial
chamber and both ventricular chambers) with multifocal fibrosis which is
sufficient enough to explain a sudden cardiac death. Given that the deceased
was young and there was no significant past medical history, primary
cardiomyopathy can be considered initially. However, microscopically there were
interstitial and replacement-type fibrous tissue deposition in the left
ventricular wall, only focal minimal increase in interstitial fibrous tissue
deposition is noted in the right ventricular wall, and lymphocytic infiltrates
with myonecrosis and cardiomyocyte dropout in the interventricular septum.
Therefore, these findings were considered as a reasonable basis to determine a
diagnosis of healing myocarditis (Figure
2). A hypertrophied heart usually presents macroscopically with
a four- chamber dilation and cardiomegaly (beyond normal weight), and
microscopically with myocyte hypertrophy and myocardial fibrosis, even though
in some subsets of cardiomyopathies, the heart weight can be within normal
limits [6,7]. Hypertrophied heart with multifocal fibrosis is a nonspecific
pathologic finding, which can be primary or secondary cardiomyopathies. In
terms of the diagnosis of secondary cardiomyopathies, the past history of
underlying diseases or drugs, macroscopic and microscopic findings of other
organs can be helpful. In this case, there was no significant history, and no
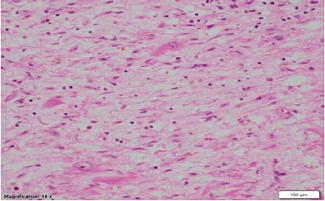
pathologic findings were observed in other organs. Etiologically, primary
cardiomyopathies can be grouped into three categories which are genetic, mixed,
and acquired. Given that the deceased was a young man without any significant
history, hypertrophic or arrhythmogenic ventricular cardiomyopathy and
myocarditis can also be considered as plausible differential diagnoses. There was
no myofiber disarray observed in the multiple sections of the heart which is a
pathognomonic finding in hypertrophic cardiomyopathy (Figure 3). Acute myocarditis typically reveals an acute inflammation,
which histologic findings should show various components of inflammatory cells
depending on each type of myocarditis. However, myocarditis typically evolves
through healing or healed stages, which characterizes myocardial fibrosis and
myocyte hypertrophy. These findings can mimic arrhythmogenic ventricular
cardiomyopathy, which may complicate a diagnosis, or may be impossible to
distinguish, especially if there is subepicardial fibrosis in the left
ventricle with fat infiltration [8-10]. Our case also revealed myocardial
fibrosis in the subepicardial third of the left ventricular myocardium (Figure 4). However, arrhythmogenic ventricular cardiomyopathy typically
reveals fibrofatty replacement with marked myocyte hypertrophy and myocardial
degeneration, in random distribution in the right and left ventricular
myocardium [5]. In our case, lymphocytic infiltrates with myonecrosis was
identified on the muscular septum. Myocardial fibrosis was identified
multifocally in the left ventricular wall and within the interventricular
septum. It was not a fibrofatty replacement and it showed a band-like
distribution within the mid myocardium as well as the subepicardial third of
the left ventricle, rather than a random distribution. Only a focal, minimal
increase in interstitial fibrous tissue deposition was noted within the right
ventricular myocardium. In addition, the remaining area of the left ventricle, which
is spared from fibrosis, revealed just mild myocyte hypertrophy. This band-like
pattern of myocardial fibrosis, sparing the right ventricle, inflammatory
infiltrates with myonecrosis, and mild myocyte hypertrophy in the remaining
myocardium strongly supported healing myocarditis. We have described a case of a sudden cardiac death in a
young man with healing myocarditis. Primary cardiomyopathy and myocarditis can
be considered in a young man with a hypertrophied heart with multifocal
fibrosis. It is especially hard to distinguish ongoing myocarditis from
arrhythmogenic ventricular cardiomyopathy. The pattern of myocardial fibrosis
and its distribution, presence of inflammatory cell infiltrates with
myonecrosis, and insignificant features of the remaining myocardium can be the
key elements to make a diagnosis of healed or healing myocarditis. We acknowledge the expert opinion and invaluable
contribution of Dr. Kristopher Cunningham MD, PhD, FRCPC, Cardiovascular and
Forensic Pathologist of Ontario Forensic Pathology Service for this case
report. Herath CJ, Ontario Forensic
Pathology Service, Department of Laboratory Medicine and Pathobiology, 25
Morton Shulman Avenue, Toronto, Ontario, Canada, Tel: 647-3291926, E-mail:
Jayantha.Herath@ontario.ca Herath CJ and Park S. Healing myocarditis
from a hypertrophied heart with multifocal fibrosis mimicking cardiomyopathy
(2019) Clinical Cardiol Cardiovascular Med 3: 14-16 Myocardial fibrosis, Forensic pathology, Ventricular arrhythmia, Subepicardial fibrosis, Cardiomyopathy.Healing myocarditis from a hypertrophied heart with multifocal fibrosis mimicking cardiomyopathy
Sohyung Park and Jayantha C Herath
Abstract
Full-Text
Introduction
Case Report

Discussion
Conclusion
Acknowledgement
References
*Corresponding author
Citation
Keywords