Research Article :
Mohammed Ahmed Bamashmos and Khaled Al-Aghbari Hyperuricemia is a metabolic problem that has
become increasingly common worldwide over the past several decades. Its
prevalence is increased in both advanced and developing countries including
Yemen. The aim of this cross sectional study was to investigate the prevalence
of hyperuricemia in sample of Yemeni adult individual and its relationship to
certain cardiovascular risk factors namely obesity, hypertension, serum
glucose, total cholesterol, high serum triglyceride, Low High Density
Lipoprotein (HDL-C) and high Low Density Lipoprotein (LDL-C). A sample of 600 adult
Yemeni people aged equal or over 18 years was randomly chosen to represent the
population living in Sanaa City during a period of two years from April 2017 to
August 2018. All the study groups undergo full clinical history and examination
includes measurement of BP and BMI, WC and the following laboratory
investigation (FBS, Basal serum uric acid level, total cholesterol, serum TG,
HDL and LDL). The prevalence of
hyperuricemia in this study was 8.8% (11,6% male and 6.4% female). The serum
uric acid level in this study was significantly correlated with age, Waist
Circumference (WC), SBP, DBP, FBS, T-cholesterol, TG and LDL but not with HDL. There is strong
relationship between serum uric acid level and other cardiovascular risk
factors. Hyperurecemia
is metabolic problem that has become increasingly worldwide over the past
several decades and its the most risk factor for gout [1]. Hyperurecimea is
defining as serum urate level greater than 6mg/dl in in women and 7mg/dl in
men, above this concentration serum urate supersaturates in body fluid and is
prone to crystallization and subsequent deposition in the tissue [2].The
association of hyperurecemia and gout with other medical condition such as
hypertension, chronic kidney diseases, dyslipidemia and other cardiovascular
diseases has been recognized for over 100 years [3]. The prevalence of
hyperurecemia has been increased in recent years, not only in advanced
countries but also in developing countries along with the development with
their economics [4]. Published population-based prevalence data of
hyperuricemia were reported in 13 of the 21 Global Burden of Diseases
(GBD) regions, and a total of 24 countries. In most part of Asia Hyperuricemia
is relatively prevalent, but in East Asia it found to be most prevalent. Lowest
percentage is seen in Papua New Guinea 1% and in Marshall Islands 85% is seen. In japan the high income Asian country Hyperuricemia has
increased by five folds in time of two decades. There is no published
population-based epidemiological studies on hyperuricemia were identified
during the specified systematic review period [5]. Hyperuricemia can be causes
by over production of urate which account of less than 10% of the cause such as
high cellular turnover, genetic error and tumor lysis syndrome or far more
commonly inefficient excretion by the kidney due to renal insufficiency of any
cause or medication that impair renal urate clearance [6]. In numerous
epidemiological studies since 1950 a positive association has been seen between
serum uric acid and cardiovascular
diseases such as ischemic heart disease and stroke [7,8]. However weather uric acid is independent risk factor for
cardiovascular disease is still disputed as several studies has suggested that
hyperurecemia is merely associated with cardiovascular disease because of
confounding factors such as obesity, hypertension, dyslipidemia, use of
diuretic and insulin resistance [9]. Obesity and central fat distribution were
associated with hyperurecemia. Patients with central obesity have greater risk
of hyperurecemia [4]. There are many researches was conducted to evaluate the
relationship between leptin hormone and the cluster of hyperurecemia in order
to identify the pathogenic mechanism associating obesity with hyperurecemia. It
was suggested that leptin could be a pathogenic factor responsible for
hyperurecemia in obese patients [10]. The strong association between hypertension and
hyperurecemia has been recognized for more than century. More than one large
epidemiological studies published over the past 7 years have found that serum
urate level predict later development of hypertension [11,12]. Experimental studies have reported that hyperurecemia induces
systemic hypertension and renal injury via activation of renin angiotensin
system and direct intery of uric acid into both endothelial and vascular smooth
muscle cells, decreased neuronal nitric oxide synthase in the juxtaglomerular
apparatus resulting in local inhibition of endothelial nitric oxide level,
stimulation of vascular smooth muscle cell proliferation and stimulation of
vasoactive and inflammatory mediators [13,14]. There is strong association
between hypertriglycemia and hyperurecemia this association could be explained
by insulin resistance as hypertriglyceridemia and hyperurecemia are suggested to
be associated with insulin
resistance syndrome [15]. The association of hypertriglyceridemia and hyperurecemia in
patients with insulin resistance syndrome could be explained by accumulation of
glycolytic intermediate and release of free fatty acid from adipose tissue
[16]. The resemblance of hyperurecemia and the metabolic syndrome has led to
the suggestion that the metabolic syndrome can be further expanded to include
hyperurecemia [17]. A prospective study in Korea suggested that higher uric
acid concentration predicted the incidence of hypertension and the development
of metabolic syndrome and hyperurecemia has been considered as component of
metabolic syndrome [18,19]. This was across sectional population based study conducted in
Sanaa city for a period of 2 years between September 2016 and September 2018 a
sample of 600 adult Yemeni people (275 male and 325 female aged ≥ 18 years) was
randomly selected from those attending Al-Kuwait University Hospital and
Consultation Clinic. All the participants in this study undergo complete clinical
history (regarding their age, occupation, habit, any history of hypertension,
diabetes mellitus, dyslipidemia and medication) Anthropometric measurement
includes measurement of height, weight, waist circumference and systolic and diastolic
blood pressure. Height was measured with tapeline to the nearest.5cm and
weight was measured with beam scale balance. Participants wore light clothing
and were asked to remove shoes, heavy outer garments Body Mass Index (BMI:
kg/m2) was calculated from measured weight and height. BMI was classified as
underweight (<18.5 kg/m2), normal (18.5-25 kg/m2), overweight (25-30 kg/m2)
and obese (>30 kg/m2) by WHO criteria [20]. Waist circumference was manually
measured on standing subjects with soft tape midway between the lowest rib and
the iliac crest. Abdominal obesity was defined as WC ≥ 90 cm (male) or WC ≥ 80
cm (female) by IDF consensus [21]. Two blood pressure recording were obtained from the right arm
of patients with slandered mercury sphygmomanometer
in a sitting position after 10 min. of rest measurement were taken in 3-5
minutes interval and the mean values were calculated. Blood pressure was
classified as normotensive (SBP<120 mmHg and DBP<80 mmHg),
pre-hypertensive (SBP: 120-139 mmHg and/or DBP: 80-89 mmHg) and hypertensive
(SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg) by the Seventh Report of the Joint
National Committee on the Prevention, Detection, Evaluation, and Treatment of
High Blood Pressure (JNC-7) [22]. The American Diabetes Association criteria
was used to classify FBG as normal glucose (FBG<5.6 mmol/L), Impaired
Fasting Glucose (IFG) (FBG ≥ 5.6 mmol/L ≤ FBG<7.0 mmol/L), and diabetic (FBG
≥ 7.0 mmol/L, Serum uric acid were measured [23]. Hyperurecemia is defined as
serum uric acid level greater than 6.0mg/|dl in women and 7.0 mg/dl in men.
Dyslipidemia was classified according to ATP III, TG: Normal<1.69 mmol/L,
Borderline high 1.69-2.26 mmol/L, High 2.26-5.65 mmol/L, Very high ≥ 5.65
mmol/L; TC: Desirable<5.17 mmol/L, Borderline high 5.17-6.24 mmol/L, High ≥
6.24 mmol/L; HDL-C: High 1.56 mmol/L, Optimal 1.03-1.56 mmol/L, Low The results were analyzed by using (Social Package of
Statistical Science) SPSS V.15 from LEAD Technologies Inc., USA. Basic
characteristics of subjects are presented as mean and slandered deviation for
quantities variables and as frequency and percent for qualitative variable. The
total participants were divided into two groups according to the sex then there
were divided into two mean groups according to serum uric acid level. The
prevalence of cardiovascular diseases risk factors among two groups were
calculated and Chi-square
test was used to detect the significance /The mean of uric acid in
different categories of separated variable were determined and the comparison
between the mean was achieved by independent t-test and one way ANOVA test. The
relationships between parameters were examined by calculating persons
correlation coefficient. For investigating for most effective factors on
hyperurecemia such as blood pressure anthropometrical and biochemical (except
UC) measurement were considered for Binary logistic regression P-value less
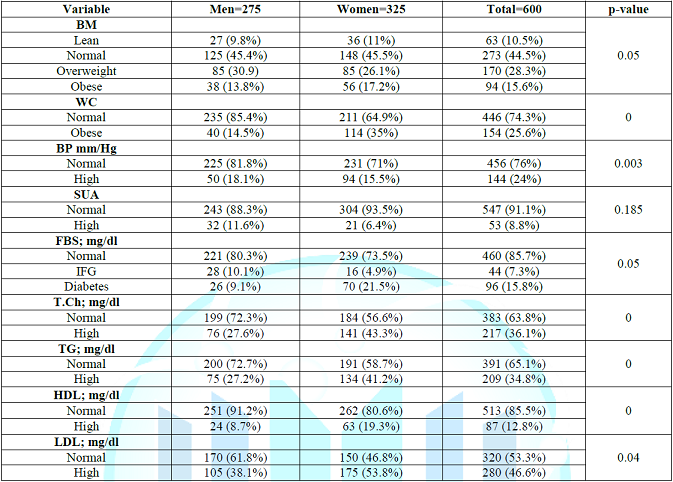
than 0.05 was considered statistically significant. A study sample includes 600 person aged between 18-83 of them
275 (45.4%) were male and 325 (54.6%) were female. The prevalence of high serum
uric acid level in the study group was 53 (8.8%) with no significant difference
between male and female (Table 1) regarding the clinical and laboratory
parameters in the study group BMI. WS, serum cholesterol, high TG, low high
density lipoprotein, high LDL and high FBS were significantly higher in women
than in men (17.2%, 35%, 43.3%, 41.2%, 19.3%, 53.8% and 26.4% vs 13.8%, 14.5%,
27.6%. 27.2%, 8.7%, 38.1% and 19.2% respectively, while the BP was
significantly higher in men than women, also there was no significant
difference between male and female regarding serum uric acid level. Hyperurecemia is increasingly common medical problem not only
in the advanced countries, but also in the developing countries. The incidence
of high serum uric acid is increased word wide with average of 20% of
population having hyperurecemia and the serum uric acid level is increased with
age. It has been described that hyperurecemia is associated with other
cardiovascular risk factors such as obesity, dyslipidemia,
hyperglycemia and hypertension [1-3]. Elevated serum uric acid levels are
commonly seen in association with glucose intolerance, hypertension and
dyslipidemia, a cluster of metabolic and hemodynamic disorders
which characterize the so-called metabolic syndrome [25-29]. To our knowledge
there is no data about the prevalence of hyperurecemia in Yemen so we decided
to carry out this research in order to know the prevalence of hyperurecemia and
its association with other cardiovascular risk factors in Yemeni population. Note: SUA-Serum
Uric Acid, FBS- Fasting Blood Sugar, IFG- Impaired Fasting Glucose, T.Ch- Total
Cholesterol, TG- Triglyceride, HDL- High Density Lipoprotein, LDL- Low Density
Lipoprotein. Table 1: Shows the clinical and laboratory characteristics of
the study group. Note: Table 2
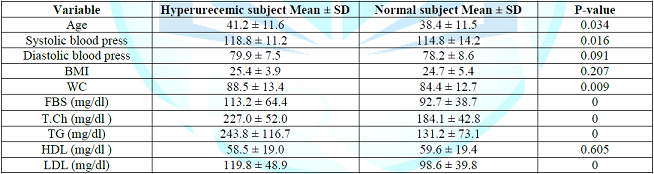
present comparing the mean of selected study parameters in relation to the uric
acid level, it shows the mean of age, SBP, WS, fasting blood glucose, total
cholesterol, TG and LDL were significantly higher within hyperurecemic study
population in comparing to those with normal serum uric acid level, there were
no significant difference in DBP, BMI and HDL between the two groups. Note: Table 3
shows the simple correlation coefficients between serum uric acid levels and
the various cardiovascular risk factors in the population. Uric acid was
significantly positively correlated with age, SBP, FBS, TG, total cholesterol,
LDL (P-value ≤ 0.01) and weak positive correlated with DBP, BMI and WC (P-value
≤ 0.05 ) while it was insignificantly correlated with HDL (P-value 0.411). The mean observations of the present study are the following;
firstly the prevalence of hyperurecemia present in a good proportion in Yemeni
peoples and it was insignificantly high in women than in men. Secondly
significant correlation between serum uric acid the various cardiovascular risk
factors were found. The prevalence of hyperurecemia in the present study was
8.8%, which is mainly near to that reported in Saudi Arabia (9.3%), Iran (8%),
Thailand (9-11%), Mexico (11%) and in Turkish (12%) which may be reflected to
similar race and environmental factors. while its lower than that found in
Columbia (26.3%). Indian (25.8%), Taiwan (30.4%) and USA (21-22%) which may be
attributed to the high economic state of this countries. Hyperurecemia was
insignificantly higher in women (7.9%) than men (6.3%) which may be explained
by high prevalence of obesity in women (BMI and WC was 17.2%, 35% in women VS
13.8% and 14.1% in in men respectively. This finding was supported by study
done in Saudi Arabia and but other studies are against this observation.
Comparing hyperurecemic subject with those with normal serum uric acid level,
those with hyperurecemia are older centrally obese, had high systolic blood
pressure, high FBS, total cholesterol, triglyceride, and LDL
[30-36] (Table 2). In this study, multiple logistic regression results have
further confirmed the association between metabolic abnormalities and high
serum uric acid, and have conducted further stratified analysis on each
metabolic abnormality-related indicator. Table 3 shows the simple correlation
coefficients between serum
uric acid levels and the various cardiovascular risk factors in the
population. Our results have shown that high serum uric acid was significantly
positively correlated with age, SBP, FBS,TG, total cholesterol, LDL (P-value ≤
0.01) and weak positive correlated with DBP, BMI and WC (P-value ≤ 0.05 ) while
it was insignificantly correlated with HDL (P-value 0.411) [35]. Elevation of the serum uric acid level has been known
associated with major cardiovascular risk factors, such as hypertension,
insulin resistance, dyslipidemia and obesity, which are hallmarks of metabolic
syndrome [36-39]. Similar to other studies, in this study, individuals with
hyperuricemia had higher prevalence of major cardiovascular risk factors,
including dyslipidemia, hypertension and overweight. Uric Acid (UA) is a known
endogenous scavenger, which provides a major part of the antioxidant capacity
against oxidative and radical injury. However, at high levels, UA can shift
from an antioxidant to a pro-oxidant factor (shuttle capacity),
depending on the characteristic of the surrounding microenvironment (e.g., UA
levels, acidity, depletion of other antioxidants, reduced Nitric Oxide (NO),
availability) [40,41]. Accordingly, high UA values have been associated with
metabolic syndrome, Cardiovascular Disease (CVD), and renal dysfunction,
involving mechanisms that favor oxidative stress, inflammation, and endothelial dysfunction.
The result of this study showed significant positive correlation were found
between serum uric acid and several component of the metabolic syndrome such as
higher WS, BP, TG and FBS (p-value ≤ 0.005) but there was insignificant
negative correlation with HDL. Several possible pathophysiological mechanisms
have been evoked to explain these associations including insulin resistance,
the use of diuretics or impaired renal function accompanying hypertension
[42-49]. Indeed the kidney seems to play an important role in the
development of the metabolic syndrome. Insulin-resistant individuals secrete
larger amounts of insulin in order to maintain an adequate glucose metabolism.
The kidney which is not insulin-resistant responds to these high insulin levels
by decreasing uric acid clearance, probably linked to insulin-induced urinary
sodium retention. Insulin resistance may increase blood pressure directly via
enhanced proximal tubular sodium reabsorption or indirectly by the sympatho-adrenal system
[43-45]. Thereby, the kidney has been implicated as the potential link between
muscle insulin resistance and compensatory hyperinsulinemia and the development
of hyperuricemia and eventually hypertension. Our study demonstrates an alarming high prevalence of
hyperurecemia among Yemeni patients that increases the burden on overstrained
Yemeni health system with uprising CVDs and other hyperurecemia related health
problems e.g. hypertension, dyslipidemia DM. There is also an urgent need to
develop strategies for prevention, detection, and treatment of hyperurecemia
that could contribute to decreasing the incidence of grave consequences such
cardiovascular disease and chronic renal diseases. 1.
Terkeltaub RA. Clinical practice Gout (2003) N Engl
Med 349. 2.
Sachs L, Batra LK and Zimmermann B. Medical implication
of hyperurecemia (2009) Med Healt Rhode Island 92: 353. 3.
Feig DIU, Kang DH and Johnson RJ. Uric acid and
cardiovascular risk (2008) N Engl Med 359: 1811-1821. 4.
Li-ying C, Wen H, Chen Z, Dai H and Ren J.
Relationship between hyperurecemia and metabolic syndrome (2007) J Zhejiang
University Sci 8: 593-598. 5.
Smith E and March L. Global Prevalence of
Hyperuricemia: A Systematic Review of Population-Based Epidemiological Studies
(2015) Am collage rheumatol 9. 6.
Andrew J, Luk M and Simkin P. Epidemiology of
hyperirecemia and gout (2005) Am collage managed care 11: s435-s442. 7.
Gertler MM, Gam SM and Levine SA. Serum uric acid
level in relation to age and physique in health and coronary heart diseases
(1951) Ann Intern Med 34: 1421-1431. https://doi.org/10.7326/0003-4819-34-6-1421
8.
Fang J and Alderman MH. Serum uric acid level and
cardiovascular mortality; The NHANES 1 epidemiology follow up study 1971-1992
(2000) JAMA 283: 2404-2410. https://doi.org/10.1001/jama.283.18.2404
9.
BurnierM and BrunnerHR. Is hyperurecemia a predictor
of cardiovascular risk? (1999) Curr Opin Nephrol Hypertens 8: 167-172. 10.
Fruehwald SB and PetersA, Kern W and Beyer J. Serum
leptin is associated with serum uric acid concentrations in humans (1999)
Metabolism 48: 677-680. https://doi.org/10.1016/s0026-0495(99)90163-4
11.
Perlstein TS, Gumieniak O and Williams GH. Uric acid
and the development of hypertension in normotensive aging study (2006)
Hypertension 48: 1013-1036. 12.
Krishnan E, Kwoh CK, Schumacher HR and Kuller L.
Hyperurecemia and incidence of hypertension among men without metabolic syndrome
(2007) Hypertension 49: 298-303. https://doi.org/10.1161/01.hyp.0000254480.64564.b6
13.
Mazzali M, Kanellis J, Han L, Feng L, et al.
Hyperurecemia induce primary arteeriopathy in rats by a blood pressure
independent mechanism (2002) Am J Physiol Renal Physiol 282: F991-F997. 14.
Johnson RJ. Kang DH and Feig D. Is there apathogenetic
rule for uric acid in hypertension and cardiovascular and renal diseases (2003)
Hypertension. 15.
Bosello E and Zamboni M. Visceral obesity and
metabolic syndrome (2000) Obes Rev 1: 47-56. 16.
Leyva F, Wingrove CS and Godsland IF. The glycolytic
pathway to coronary herat diseases; ahypothesis (1998) Metabolism 47: 6657-662. 17.
Klein BEK, Klein R and Lee KE. Components of metabolic
syndrome and risk of cardiovascular diseases and diabetes in Beaver Mam (2002)
Diabetes Care 25: 1790-1794. https://doi.org/10.2337/diacare.25.10.1790
18.
Yoo TW, Sung KC, Shin HS, Kim BS, Lee HM, et al.
Relationship between serum uric acid concentration and insulin resistance and
metabolic syndrome (2005) Circ J 9: 928-933. https://doi.org/10.1253/circj.69.928
19.
Onat A, Uyarel H, Hergenc G, Karabulut A, Albayrak S,
et al. Serum uric acid is a determinant of metabolic syndrome in
population-based study (2006) Am J Hypertens 19: 1055-1062. https://doi.org/10.1016/j.amjhyper.2006.02.014
20.
Allison DB, Downey M, Atkinson RL, et al. Obesity as a
disease: a white paper on evidence and arguments commissioned by the Council of
the Obesity Society (2008) Obesity (Silver Spring) 16: 1161-1177. https://doi.org/10.1038/oby.2008.231
21.
Hou X, Lu J, Weng J, Ji L, Shan Z, Liu J, Billington
JC, Bray AG, et al. Impact of waist circumference and body mass index on risk
of cardio metabolic disorder and cardiovascular disease in Chinese adults: a
national diabetes and metabolic disorders survey (2013) PLoS One 8: e57319. https://doi.org/10.1371/journal.pone.0057319
22.
European Society of Hypertension-European Society of
Cardiology Guidelines Committee: Guidelines for the management of arterial
hypertension (2003) J Hypertens 21: 1011-1053. https://doi.org/10.1097/00004872-200306000-00001
23.
American Diabetes Association. Standards of Medical
Care in Diabetes-2011 (2011) Diabetes Care 34: S11-S61. https://dx.doi.org/10.2337%2Fdc11-S011
24.
Expert panel on detection, evaluation and treatment of
high blood cholesterol in adults. Executive summary of the third report of the National
Cholesterol Education Program (NCEP) (Adult Treatment Panel III) (2001) JAMA
285: 2486-2497. https://doi.org/10.1001/jama.285.19.2486
25.
Bonora E, Targher G, Zenere MB, Saggiani F, Cacciatori
V, et al. Relationship of uric acid concentration to cardiovascular risk
factors in young men. Role of obesity and central fat distribution. The verona
young men atherosclerosis risk factors study (1996) Int J Obes Relat Metab
Disord 20: 975-980. 26.
Zavaroni I, Mazza S, Fantuzzi M, DallAglio E, Bonora
E, et al. Changes in insulin and lipid metabolism in males with asymptomatic
hyperuricemia (1993) J Intern Med 234: 25-30. 27.
Vuorinen-Markkola H and Yki-Järvinen H. Hyperuricemia
and insulin resistance (1994) J Clin Endocrinol Metab 78: 25-29. https://doi.org/10.1210/jcem.78.1.8288709
28.
Facchini F, Ida Chen Y-D, Hollenbeck CB and Reaven GM.
Relationship between resistance to insulin-mediated glucose uptake, urinary
uric acid clearance and plasma uric acid concentration (1991) JAMA 266:
3008-3011. http://dx.doi.org/10.1001/jama.1991.03470210076036
29.
Reaven GM. The kidney: an unwilling accomplice in
syndrome X (1997) Am J Kidney Dis 30: 928-931. https://doi.org/10.1016/S0272-6386(97)90106-2
30.
Al-arfa AS. Hyperurecemia in Saudia Arabia (2001)
Rhaumatol Int 20: 61-64. https://doi.org/10.1007/s002960000076
31.
Smith E and March L. Global Prevalence of
Hyperuricemia: A Systematic Review of Population-Based Epidemiological Studies
(2015) Arthritis Rheumatol 67. 32.
Sari I, Akar S, Pakoz B and Sisman AR. The prevalence
of hyperurecemia in an urban population, Izmer Turkey (2009) Rheumatol Int 29:
869-874. http://dx.doi.org/10.1007/s00296-008-0806-2
33.
Lombo B, Melo AA, Benavides J and Lopez M. Prevalence
of Hyperuricemia and Cardiovascular Risk Factors in a Population from Colombia
(2010) World congress of cardiology, China, 122. 34.
Billa G, Dargad R and Mehta A. The prevalence of hyperurecemia
in indian subject attending hyperurecemia screening program -A retrospective
study (2018) J the association of phys ind 66. 35.
Qian Yu, Hsi-che Shen, Yi-chun Hu, Yu-fen Chen and
Tao-hsin Tung. Prevalence and Metabolic Factors of Hyperuricemia in an Elderly
Agricultural and Fishing Population in Taiwan (2017) Arch Rheumatol 32:
149-157. https://doi.org/10.5606/archrheumatol.2017.6075
36.
Masuo K, Kawaguchi H, Mikami H, Ogihara T and Tuck ML.
Serum uric acid and plasma norepinephrine concentrations predict subsequent
weight gain and blood pressure elevation (2003) Hypertension 42: 474-480. https://doi.org/10.1161/01.hyp.0000091371.53502.d3
37.
Sundström J, Sullivan L, DAgostino RB, Levy D, William
B, et.al. Relations of serum uric acid to longitudinal blood pressure tracking
and hypertension incidence (2005) Hypertension 45: 28-33. 38.
Li C, Hsieh MC and Chang SJ. Metabolic syndrome,
diabetes, and hyperuricemia (2013) Current opinion in rheumatology 25:210-216. https://doi.org/10.1097/bor.0b013e32835d951e
39.
Puig JG and Martinez MA. Hyperuricemia, gout and the
metabolic syndrome (2008) Current opinion in rheumatology 20:187-191. https://doi.org/10.1097/bor.0b013e3282f4b1ed
40.
Ames BN, Cathcart R, Schwiers E and Hochstein P. Uric
acid provides an antioxidant defense in humans against oxidant- and
radical-caused aging and cancer: A hypothesis (1981) Proc Natl Acad Sci 78:
6858-6862. https://doi.org/10.1073/pnas.78.11.6858
41.
Pingitore A, Lima GP, Mastorci F, Quinones A, Iervasi
G, et.al. Exercise and oxidative stress: Potential effects of antioxidant
dietary strategies in sports (2015) Nutrition 31:916-922. https://doi.org/10.1016/j.nut.2015.02.005
42.
Gliozzi M, Malara N, Muscoli S and Mollace V. The
treatmentof hyperuricemia (2015) Int J Cardiol. 43.
Battelli MG, Polito L and Bolognesi A. Xanthine
oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress
(2014) Atherosclerosis 237: 562-567. https://doi.org/10.1016/j.atherosclerosis.2014.10.006
44.
Billiet L, Doaty S and Katz DJ. Review of
hyperurecemia as marker of metabolic syndrome (2014) ISRN Rhaumatology Pp: 1-7.
http://dx.doi.org/10.1155/2014/852954
45.
Reaven GM. The kidney: an unwilling accomplice in
syndrome X (1997) Am J Kidney Dis 30: 928-931. https://doi.org/10.1016/s0272-6386(97)90106-2
46.
Rathmann W, Funkhouser E, Dyer AR and Roseman JM.
Relations of hyperuricemia with the various components of the insulin
resistance syndrome in young black and white adults: the CARDIA study. Coronary
Artery Risk Development in Young Adults (1998) Annals Epidemiol 8: 250-261. https://doi.org/10.1016/s1047-2797(97)00204-4
47.
Agamah ES, Srinivasan SR, Webber LS and Berenson GS.
Serum uric acid and its relations to cardiovascular disease risk factors in
children and young adults from a biracial community: The Bogalusa Heart Study
(1991) J Lab Clin Med 118: 241-249. 48.
Lee J, Sparrow D, Vokonas PS, Landsberg L and Weiss
ST: Uric acid and coronary heart disease risk: evidence for a role of uric acid
in the obesity-insulin resistance syndrome. The Normative Aging Study (1995) Am
J Epidemiol 142: 288-294. https://doi.org/10.1093/oxfordjournals.aje.a117634
49.
Bruno G, Cavallo-Perin P, Bargero G, Borra M, Calvi V,
et.al. Prevalence and risk factors for micro-and macroalbuminuria in an Italian
population-based cohort of NIDDM subjects (1996) Diabetes Care 19: 43-47. https://doi.org/10.2337/diacare.19.1.43 Mohammed Ahmed Bamashmos, Associate Professor of
internal medicine and Endocrinology, Sanaa University, Yemen, E-mail: mohbamashmoos@yahoo.com Bamashmos AM and
Al-Aghbari K. Prevalence of hyperuricemia and its association with other
cardiovascular risk factors in adult Yemeni people of Sanaa city (2019)
Clinical Cardiol Cardiovascular Med 3: 10-14. Hyperurecemia, Dyslipidemia, BMI, FBS.Prevalence of Hyperuricemia and its Association with Other Cardiovascular Risk Factors in Adult Yemeni People of Sana City
Abstract
Objective: Full-Text
Introduction
Material
and method
Results
Discussion

Conclusion
References
*Corresponding author:
Citation:
Keywords