Research Article :
Gary L Murray and Joseph Colombo To review our studies of the ease and
importance of Parasympathetic and Sympathetic (P&S) measures in managing
cardiovascular patients. The autonomic nervous
system is responsible for the development or progression of Hypertension (HTN),
orthostasis, Coronary Disease (CAD), Congestive Heart Failure (CHF) and
arrhythmias. Finally, new technology provides us with rapid, accurate P and S
measures critically needed to manage these patients much more successfully. Using the ANX 3.0 autonomic
monitor, P&S activity was recorded in 4 studies: 163 heart failure patients
in total, mean follow-up (f/u) 12-24.5 months; 109 orthostasis patients, f/u
2.28 years and 483 patients with risk factors or known HTN, CAD or CHF, f/u
4.92 yrs. All were on guideline-driven therapy. 59% of CHF patients had
dangerously high Sympathovagal Balance (SB) or Cardiac Autonomic Neuropathy
(CAN) and Ranolazine markedly improved 90% of these, improved left ventricular
ejection fraction in 70% of patients on average 11.3 units, and reduced Major
Adverse Cardiac Event (MACE) [Acute Coronary Syndromes (ACS), death, acute CHF,
Ventricular Tachycardia/Ventricular Fibrillation (VT/VF)] 40%. 66% of
orthostatic patients corrected with (r) Alpha Lipoic Acid ([r]ALA);
non-responders had the lowest S-tone. In the 483 patient study, SB>2.5 best
predicted MACE when compared to nuclear stress and echocardiography (sensitivity
0.59 or 7.03 [CI (Confidence Interval) 4.59-10.78], specificity 0.83, positive
predictive value 0.64 and negative predictive value 0.80). Parasympathetic and
sympathetic measures significantly improve care of cardiovascular patients. High Sympathetic (S) tone and CAN; defined as critically low
resting Parasympathetic [P] tone, P<0.10 beats per minutes 2 [bpm2] have
been associated with ACS, CHF, malignant ventricular arrhythmias,
and increased mortality [1-8]. Good P-tone is cardio-protective [9]. Despite
knowing this, we dont use (P&S) measures routinely, if at all, to help risk
stratify and medically manage our patients. Historically, the reason for this
may be due to the difficulty generalizing non-invasive autonomic measures to
typical clinical populations. Heretofore, non-invasive measures of autonomic
activity, including those based on beat-to-beat cardiac activity, are all
measures of only total autonomic activity, forcing assumption and approximation
to theorize P&S activity. However, weve found the user-friendly accurate,
relatively inexpensive, easily mastered ANX 3.0 P&S monitor (formerly ANSAR
Medical Technologies, Inc., now TMCAMS, Inc., Atlanta, GA, USA) assessment
quite valuable. The P&S Monitor is based on technology developed, validated
and verified by the first joint Bio-Medical Engineering program group from MIT
(Massachusetts Institute Technology) and Harvard [10-14]. This article briefly
reviews some of the studies we have completed as well as mentioning ongoing and
future trials. High S-tone and low P-tone at rest is relative and assessed
by (SB: SB=[resting S]/[resting P; normal is 0.4 Symptoms and P&S responses to stimuli help to
differentiate catecholaminergic from angiotensin effects, for example. High SB
may be medication induced such as with excessive utilization of inhaled beta-2
agonists for pulmonary disorders [16-18]. Beta-adrenergic and alpha-adrenergic
blockers, angiotensin-blockers [ACE-Is (Angiotensin Conversion
Enzyme Inhibitor) and ARB (Angiotensin Receptor
Blocker)] are known to reduce resting S-tone. Implanted cardiac devices and
cardiac rhythm therapy also effect the sympathetics by forcing them to entrain
to the therapy. Low SB, indicating (resting) Parasympathetic Excess
(PE) is associated with depression, syncope, excess-gut motility, and is
relieved with very low-dose anti-cholinergics. P-tone is a measure of the net,
cumulative result of both nicotinic and muscarinic receptors. When treating the
symptoms of these disorders, titrating to normalize SB is a goal. There are also proper dynamic or challenge P&S balances.
Challenge imbalances may lead to resting imbalances and complicating or
confounding resting imbalances. For example, upon assuming a head-up posture
(e.g., standing) the proper dynamic balance is a slight decrease in P-tone
quickly followed by a modest increase S-tone. This defeats the effect of
gravity causing a shift in blood to the lower extremities and vasoconstricts
the lower vasculature to support standing. A decrease in S-tone at this time
(Sympathetic Withdrawal-SW) is associated with orthostatic dysfunction and may
cause secondary, high, resting BP as a compensatory response to the decrease in
BP associated with orthostatic dysfunction. Typically, the high resting BP (Blood Pressure) is
considered the primary and treated as such, yet the patients become more
lightheaded and then become non-compliant. This is because the medication induced lower resting
pressure, which results in poor diastolic coronary and brain perfusion caused
by the decline in standing BP, and the patients body defeats the therapy to
maintain proper perfusion. Low prolonged coronary diastolic
pressure (and associated perfusion) due to SW with prolonged, high systolic
pressure, as measured by high resting BP, may lead to heart failure. P&S
monitoring helps to document these complications and guide therapy. Another
possible imbalance from the stand example is a challenge PE [15]. PE may also
lead to secondary SE and confound the treatment of high BP, for example.
Challenge PE is associated with difficult to control BP, blood glucose, various
hormone levels, increased weight, difficult to describe pain syndromes
(including chronic
refractory pain syndrome (CRPS)), unexplained arrhythmia (palpitations),
seizures, temperature dysregulation (both response to heat or cold and sweat
responses), and symptoms of depression or anxiety, fatigue, exercise
intolerance, sex dysfunction, sleep or GI disturbance, lightheadedness,
cognitive dysfunction or Brain Fog or frequent headache or migraine [19].
Challenge PE may also be treated with very low-dose anti-cholinergics, or if
heart disease, high BP, or some other form of SE, PE may be treated with the
double cocktail: carvedilol whose central alpha effect lowers PE. A better
understanding of P&S pathophysiology provides more information to reduce
morbidity and mortality risk and improve patient outcomes [15]. The ANX 3.0 P&S function monitor (hereafter designated
P&S Monitor) computes simultaneous, independent measures of P&S
activity based on continuous time-frequency analyses of Heart Rate Variability
(HRV) with concurrent time-frequency analyses of Respiratory Activity (RA). The
following variables were recorded (although not all are detailed in the results
section): 5 min. seated resting BP and P&S activity (measured as
Respiratory Frequency area [RFa] and Low-Frequency area [LFa] respectively);
Exhalation/Inhalation (E/I) ratio and RFa were computed in response to 1 min.
of deep breathing (paced at 6 breaths/min); Valsalva ratio and LFa & RFa
were computed in response to a short series of Valsalva maneuvers (10 to 15
sec. each); and HR, BP, LFa, RFa and 30:15 ratio were computed in response to 5
min. of head-up postural change (quick stand followed by quiet 5 min. standing)
[10-14]. Sympathovagal Balance is computed as LFa/RFa (reported means
are averages of ratios, not ratio of averages). P-activity (RFa) was defined as
the spectral power within a 0.12 Hz-wide window centered on the Fundamental Respiratory
Frequency (FRF) in the HRV spectrum. FRF was identified as the modal peak
from the time-frequency analysis of RA. Effectively, FRF is a measure of vagal
outflow as it affects the heart, as in Respiratory Sinus Arrhythmia (RSA).
S-activity (LFa) was defined as the remaining spectral power, after computation
of RFa in the low-frequency window (0.04-0.15 Hz of the HRV spectrum) [10-14].
The 30:15 ratio is the ratio of the 30th R-R interval after a quick head-up
postural change (standing) to the 15th R-R interval after standing. The 30:15 ratio
reflects the reflex bradycardia after standing that is dependent on sympathetic
vasoconstriction. The Valsalva ratio is the ratio of the longest R-R interval
to the shortest R-R interval during a 15 sec. Valsalva maneuver. The E/I ratio
is the ratio of the heart beat interval during peak exhalation over that during
peak inhalation during paced breathing. The E/I ratio is a threshold measure of
more or less Vagal (P) tone, as are the 30:15 and Valsalva ratios. In the first study, statistics, including means, standard
deviations, and student t-tests, were performed under SPSS v 14.1. Student
t-tests were performed as 2-tailed with equal variance. Significance values
were determined on the null hypothesis that the pre- and post-treatment P&S
values were equal. In the second study, continuous data were assessed for
normality with normally distributed data and analyzed using Student t-tests.
Non-normally distributed data were assessed using a Mann-Whitney test.
Dichotomous data were analyzed using the Chi-square test or Fishers-Exact Test.
We determined that 50 patients per group were needed to have a sufficient
sample size using an alpha of 0.05, difference of means of 6 units and expected
standard of deviation of 15 units with a power of 80%. All statistics were
performed under SPSS v 1.4. Student t-tests were performed as two-tailed with
equal variance. Significance values were determined on the null hypothesis that
pre- and post- treatment values were equal. In our third study, Receiver
Operating Characteristic (ROC) analysis was determined. SB>2.5 and (Left
Ventricular Ejection Fraction) LVEF<0.34 best predicted major cardiac events
(MACE: acute coronary syndromes, acute CHF, malignant ventricular arrhythmias,
cardiovascular death). The p-value of a SB>2.5 vs. LVEF<0.34 or
reversible defect(s) on Myocardial
Perfusion Imaging (MPI) was computed by uncorrected chi-square test. In our
fourth study, continuous data were assessed for normality with normally
distributed data using Student t-tests and non-normally distributed data using
a Mann-Whitney U test. Dichotomous data were analyzed using the chi-square test
or Fishers exact test. A p-value of 0.05 or less was considered significant.
Student t-tests as two-tailed with equal variance. Significance values were determined
on the null-hypothesis that the pre- and post-treatment values are equal. All
patients signed informed consents. Congestive Heart
Failure In our first study, 54 ACC/AHA (American College
Cardiology/American Heart Association) guideline-treated chronic CHF patients
[54% HFrEF (Heart Failure Reduced Ejection Fraction), 46% HFpEF (Heart Failure
Preserved Ejection Fraction)] were randomized to adding Ranolazine
(RANCHF-Ranolazine-Treated Heart Failure) vs. continued usual care (NORANCHF-No
Ranolazine- Treated Heart Failure) [24]. Demographics between these groups
matched well; the mean beta blocker dose was higher in the NORANCHF cohort. 59%
of the patients in each group initially had high SB, CAN or both. At 1 year,
94% of RANCHF patients improved P&S measures; 88% normalized high SB and
corrected CAN. Only 50% of NORANCHF patients improved (p=0.056). Individually, only 18% of NORANCHF patients normalized high
SB vs. 83% of RANCHF (p=0.013). Four NORANCHF patients (15%) demonstrated SB
responses that became abnormally high. At 1 year, resting P-activity was 0.50
bpm2 in RANCHF patients vs. 0.38 bpm2 in NORANCHF (p=0.004). Improvement of
P&S measures in RANCHF patients were independent of Brain Natriuretic Peptide
(BNP) and impedance cardiogram results, suggesting a direct effect of RAN on
P&S function. This was confirmed by similar improvements in P&S
measures in a 30 patient control group without known cardiac disease that had
initial CHF-like profiles. In our second study, 109 ACC/AHA guideline-treated (New York
Heart Association) NYHA class 2-4 chronic CHF patients (84 HFrEF, 25 HFpEF; 54
RANCHF, mean follow-up 24.5 mo.; 55 NORANCHF, mean follow-up 22.8 mo., were
matched for age, gender, and history [25]. 98% of patients took a beta blocker
(slightly higher dose in NORANCHF); HFpEF RANCHF patients had more patients
with HTN and chronic renal insufficiency. 70% of RANCHF patients increased LVEF
an average 11.3 units (p=0.018 for HFrEF RANCHF, initial mean LVEF 0.30); LVEF
in NORANCHF patients decreased 1 unit from initially 0.30. RAN MACE (cardiac death, acute
CHF, VT/VF) occurred in 31.5% vs. 38.2% MACE in NORANCHF (an 18% reduction).
Again, RAN improved P&S measures. SB decreased in RANCHF (p=0.019) while
increasing in NORANCHF patients (p=0.039). In the total population, final SB
was 3.5 in MACE patients vs. 2.28 in patients without. This led us to do our
third study. In our third study (unpublished data) we followed 483 patients for
a mean of 4.92 yr. (127 with CAD risk factors, 224 with CAD, 132 with chronic
CHF). We compared SB>2.5 to reversible myocardial imaging defect(s) or
LVEF<0.34 as a predictor of MACE (ACS, acute CHF, VT/VF, cardiac death). SB
independently outperformed them (p=0.001) with a sensitivity of 0.59, OR=7.03
(CI: 4.59-10.78), specificity of 0.83, PPV=0.64, and NPV=0.80. 31% of patients
had a SB>2.5. There were 3 patterns of high SB (P&S measures taken every
6 mo.): acute, chronic, and intermittent. An acutely high SB (20%) is the most
ominous. In our fourth study, in a cohort of 109 patients with low
standing S-response (known as Sympathetic Withdrawal as opposed to the normal
increase in S-activity with stand), 29 were found with Neurogenic Orthostatic
Hypotension (NOH, fall in Standing BP (sBP) of at least 20/10 mmHg), and 60
with Neurogenic
Orthostatic Intolerance (NOI, fall in sBP of -6 to -19 mmHg) [26]. Both
groups were given (r) alpha lipoic acid, at a mean dose range of 993-1500 mg/d.
A third, control, group included 20 patients with either NOH or NOI. All
patients were followed for a mean of 2.28 yr. 66% of NOH patients responded
(standing change in BP ranged from -28/-10 mmHg to 0/+2 mmHg [p=0.0129 for
systolic, p=0.0456 for diastolic pressure changes]). 67% of NOI patents
responded as well (standing change in BP ranged from -9/+1 mmHg to +6/+2 mmHg;
[p ≤ 0.001 for systolic, ns for diastolic, pressure changes]). The control
group had no changes in BP. If maintaining a diastolic BP at least 60 mmHg to
preserve coronary perfusion were taken into account, 88% of patients would be
responders. Although all patients treated with (r)ALA increased their
S-response to stand, responsiveness depended upon the resting S-tone: those
with the lowest resting S-tone (indicating advanced autonomic dysfunction)
responded the least. Sympathovagal Balance SB>2.5 increases the odds of suffering MACE seven-fold.
Dutifully prescribing ACC/AHA and (Joint National Committee) JNC 8 guidelines
for prescribing beta blockers for chronic HFrEF, CAD, and hypertension is
insufficient to insure optimal SB, which likely plays a significant role in the
continued disturbing rates of MACE in our patients. In fact, death rate per
100,000 treated hypertensives is increasing. Now we may and should measure SB,
and adjust pharmacologic therapy accordingly. Ranolazine reduces SB and MACE in
chronic CHF, likely by its effect on cardiac sodium channel 1.5 and P&S
sodium channel 1.7 [24,25]. We were the first to report Ranolazine reduces ACS
in CAD [27]. Sympathetic
Withdrawal and Orthostatic Dysfunction A resulting increase in systolic pressure over 130 mmHg
(resting) may produce pulse pressures (>70 mmHg) that are associated with
poor prognoses. Prolonged, this condition may precipitate, and certainly may
exacerbate, heart
failure. In this way SW may be associated with heart failure. Without a means
of recognizing SW, therapy would typically be directed to reducing systolic
pressure and thereby pulse pressure. However, this may exacerbate the
orthostatic dysfunction and exacerbate coronary hypo-perfusion, as well as lead
to non-compliance, or unstable or difficult to manage BP as the body attempts
to maintain coronary and brain perfusion. Similarly, if the orthostatic drop in
BP is treated as the primary, as with vasopressors, it may further increase
systolic pressure, increasing pulse pressure, thereby exacerbating heart
failure as well. Cardiovascular
Autonomic Neuropathy ·
If CAN with high
SB is demonstrated, consider sympatholytics based on history, titrated against
normalizing SB and thereby normalize mortality risk. Choice of sympatholytic
treatment is based on patient history. For example, if BP is high then consider
anti-hypertensives,
or if BP is normal to low or HR is high, then consider beta-blockers. As with
diabetics, Carvedilol is often the preferred beta-blocker for CAN with high SB. ·
If CAN with low
SB is demonstrated, consider low-dose anti-cholinergics (very low-dose anti-depressants-low-dose
to minimize morbidity risks), depending on other medical history, titrate to
normalize SB and thereby normalize mortality risk. Choice of anti-cholinergic
is based on patient history. For example if BP is high then consider very
low-dose SSRI, or if BP is normal to low then consider very low-dose SSRI or
tri-cyclic. ·
If CAN is
present with normal resting SB with a recent cardiac work-up, then mortality
risk is normal, and the resting autonomic state of the patient is well managed.
Any (resting) abnormality may be due to end-organ dysfunction. If there has not
been a recent cardiac work-up, then one is recommended. For chronic patients, CAN has been found to carry the same
50% increase in the five-year mortality rate as in diabetics [29,30]. More
recently, some data suggests that CAN represent a 50% increase in the two-year
mortality rate. In addition to geriatric patients, CAN may be normal for
post-MI, post-CABG, and CHF patients, as well as other chronic diseases. CAN is
associated with other risk factors including: ·
Low ejection
fraction ·
Poor cardiac
output ·
Arrhythmias ·
Cardiomyopathies
including chronic heart failure ·
Poor
circulation, coronary artery disease with or without angina ·
Greater
mortality and ·
Greater
morbidity including silent myocardial infarction and early cardiac death. Often, very low P-activity (CAN) leads to the need for an
implanted cardiac device [1,31-44]. P&S Monitoring was chosen for two reasons. First, P&S
Monitoring includes spectral analyses based on the time-frequency analysis
technique of Continuous
Wavelet Transforms (CWT), rather than the frequency-only analysis technique
of the Fast Fourier Transforms (FFT). Although including short-term FFT is
accurate for stationary signals, it results in a compromise in time and
frequency resolution because fixed length windows are analyzed. Therefore, the
FFT (including the short-term FFT) involves are two weak assumptions: the
P&S signals are not stationary (even at rest or during quiet standing) and
the time-frequency compromise is not static in addition to the fact that it is
a compromise. The P&S-tone values from P&S Monitoring are computed from
nonstationary, continuous, independent RA and HRV signals. CWT permits
automatic adjustment of the window length to the features of the signal. As a
result, time-frequency resolution is superior to all prior HRV studies [11-14].
Second, P&S Monitoring is the only non-invasive technique that
(mathematically) independently and simultaneously quantifies P-activity without
assumption and approximation. Other autonomic measures based on beat-to-beat
cardiac activity (e.g., HRV, beat-to-beat BP and Pulse Wave Velocity measures)
assume that P-activity is always located within the 0.15-0.40 Hz frequency
range (a wide window to improve the capture of P-activity). Instead, P&S
Monitoring measures a second, independent measure of P&S activity: RA using
impedance plethysmography. The first measure is of the heart (HRV); the second
measure is of the lungs (RA). While it is true that RSA is generated from RA (via pulmonary
baroreceptors and the Vagus Nerve), measuring RA is not a direct measure of
P-activity. RA is a second independent measure of the autonomic nervous system
and therefore fully satisfies the algebraic requirement necessary to fully
characterize a system with two independent components. With these two
independent measures as verified and validated by the MIT/Harvard team, P&S
Monitoring localizes and quantifies P-activity, and thereby S-activity, over
the period of observation without the need for assumption and approximation.
Conceptually, the P&S Monitoring process, in effect, measures RSA even when
it is not possible to visualize it from the cardiogram. Given that RSA is
purely parasympathetic in etiology, conceptualizing the measurement of RA as a
measure of RSA helps to understand the process that provides a direct measure
of P-activity. The process is based on the measure of the FRF [11-14]. For
example, if the patients respiratory rate (FRF) is slow, P could be contained
within the low frequency range (0.04-0.15 Hz), e.g., S-range of HRV. The low
frequency range represents S-activity as modulated by P-activity [10,11]. Slow
respiration leads to higher low frequency HRV activity misinterpreted as
increased S-response unless FRF is determined. For the first time, simultaneous
time-frequency analysis of HRV and RA accurately identifies P, unscrambling S
& P activity. This technological breakthrough allowed us to correctly
measure SB and CAN. P&S abnormalities, including high SB, CAN, and low S-responses
to standing (head-up postural change) are common. They cause and contribute to
the mortality, morbidity, and cost of medical care, including for CAD, chronic
CHF and NOH. Despite our ability to easily diagnose and address these P&S
abnormalities, we seldom, if ever, do. Our patients deserve better. Hypertension It may be that treating the abnormal P-excess and normalizing
P-activity, may organically normalize the S-response and thereby normalize BP.
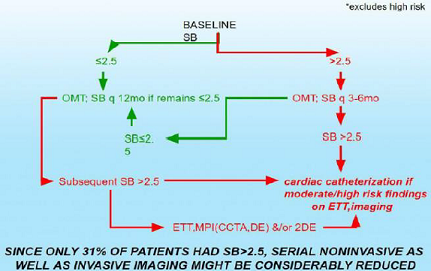
We plan to compare P&S-assisted therapy to JNC 8 (Figure 1). Coronary Disease Figure 2: Stable PTS with risk factors for CAD. Figure 3: Stable CAD, CHF PTS Baseline ETT, MPI (CCTA, DE)
&/or 2DE*. 1. Vinik
A and Ziegler D. Diabetic cardiovascular autonomic neuropathy (2007) Circulatn
115: 387-397. https://doi.org/10.1161/circulationaha.106.634949 2. Tomaselli
G and Zipes D. What causes sudden death in heart failure? (2004) Circ Res 95:
754-763. https://doi.org/10.1161/01.res.0000145047.14691.db
3. Maser
R, Mitchell B, Vinik A and Freeman R. The association between cardiovascular
autonomic neuropathy and mortality in individuals with diabetes: a
meta-analysis (2003) Diabetes Care 26: 1895-1901. https://doi.org/10.2337/diacare.26.6.1895
4. Watanabe
J, Shinozaki T, Shiba N, Fukahori k, Koseki Y, et al. Accumulation of risk
markers predicts the incidence of sudden death in patients with chronic heart
failure (2006) Eur J Heart Fail 8: 237-242. https://doi.org/10.1016/j.ejheart.2005.08.003
5. Curtis
B and OKeefe J. Autonomic tone as a cardiovascular risk factor: the dangers of
chronic fight or flight (2002) Mayo Clinic Proc 72: 45-54. https://doi.org/10.4065/77.1.45
6. McCance
A, Thompson P and Forfar J. Increased cardiac sympathetic nervous activity in
patients with unstable coronary heart disease (1993) Eur Heart J 14: 751-757. https://doi.org/10.1093/eurheartj/14.6.751
7. Manfrini
O, Morgagni C, Pizzi C, Fontana F and Bugiardini R. Changes in autonomic nervous
system activity: spontaneous versus balloon-induced myocardial ischemia (2004)
Eur Heart J 25: 1502-1508. https://doi.org/10.1016/j.ehj.2004.03.019
8. Akuttsu
Y, Kaneko K, Kodama Y, Suyama J, Shinozuka A, et al. Significance of cardiac
sympathetic nervous system abnormality for predicting vascular events in
patients with idiopathic paroxysmal atrial fibrillation (2010) Eur J Nucl Med
Mol Imaging 37: 742-749. https://doi.org/10.1007/s00259-009-1322-7
9. Abe
M, Iwaoka M, Nakamura T, Kitta Y, Takano H, et al. Association of high levels
of plasma free dopamine with future coronary events in patients with coronary
artery disease (2007) Circ J 71: 688-692. https://doi.org/10.1253/circj.71.688
10. Aysin
B, Colombo J and Aysin E. Comparison of HRV analysis methods during orthostatic
challenge: HRV with respiration our without? (2007) 29th Int Conf
IEEE EMBS Lyon, France. https://doi.org/10.1109/iembs.2007.4353474
11. Akselrod
S, Gordon D, Ubel FA, Shannon DC, Berger AC, et al. Power spectrum analysis of
heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular
control (1981) Sci 213: 220-222. https://doi.org/10.1126/science.6166045
12. Akselrod
S, Gordon D, Madwed J, Snidman N, Shannon D, et al. Hemodynamic regulation :
Investigation by spectral analysis (1985) Am J Physiol 249: H867-H875. https://doi.org/10.1152/ajpheart.1985.249.4.h867
13. Akselrod
S, Eliash S, Oz O and Cohen S. Hemodynamic regulation in SHR: investigation by
spectral analysis (1987) Am J Physiol 253: H176-H183. https://doi.org/10.1152/ajpheart.1987.253.1.h176
14. Akselrod
S. Spectral analysis of fluctuations in cardiovascular parameters: a
quantitative tool for the investigation of autonomic controls (1988) Trends
Pharmacol Sci 9: 6-9. https://doi.org/10.1016/0165-6147(88)90230-1
15. Colombo
J, Arora RR, DePace NL and Vinik AI. Clinical Autonomic Dysfunction: Measurement,
Indications, Therapies, and Outcomes (2014) Springer Science, New York, USA. 16. Salpeter
SR. Cardiovascular safety of β2-adrenoceptor agonist use in patients with
obstructive airway disease: a systematic review (2004) Drugs Aging 21: 405-414.
https://doi.org/10.2165/00002512-200421060-00005
17. Cazzola
M, Matera MG and Donner CF. Inhaled β2-Adrenoceptor Agonists: Cardiovascular
Safety in Patients with Obstructive Lung Disease (2005) Drugs 65: 1595-1610. https://doi.org/10.2165/00003495-200565120-00001
18. Salpeter,
Shelley R. et al. Cardiovascular Effects of β-Agonists in Patients with Asthma
and COPD: A Meta-Analysis (2004) CHEST 125: 2309-2321. https://doi.org/10.1378/chest.125.6.2309
19. Tobias
H, Vinitsky A, Bulgarelli RJ, Ghosh-Dastidar S and Colombo J. Autonomic nervous
system monitoring of patients with excess parasympathetic responses to
sympathetic challenges-clinical observations (2010) US Neurology 5: 62-66. https://doi.org/10.17925/usn.2010.05.02.62
20. Triposkiadis
F, Karavannis G, Skoularigis J, Louridas G and Butler J. The sympathetic nervous
system in heart failure physiology, pathophysiology, and clinical implications
(2009) J Am Coll Cardiol 54: 1747-1762. 21. Bibevski
S and Dunlap M. Evidence for impaired vagus nerve activity in heart failure
(2011) Heart Fail Rev 126: 129-133. https://doi.org/10.1007/s10741-010-9190-6
22. Martignani
C, Diemberger J, Nanni C, Biffi M, Ziacchi M, et al. Cardiac resynchronization
therapy and cardiac sympathetic function (2015) Eur J Clin Invest 45: 792-799. https://doi.org/10.1111/eci.12471
23. Del
Mazumder D, Kass D, ORourke B and Tomaselli G. Cardiac resynchronization
therapy restores sympathovagal balance in failing heart by differential
remodeling of cholinergic signaling (2015) Circ Res 116: 1691-1699. https://doi.org/10.1161/circresaha.116.305268
24. Murray
G and Colombo J. Ranolazine improve autonomic balance in heart failure when
added to guideline-driven therapy (2014) Heart International 9: 1-7. https://doi.org/10.5301/heartint.5000215
25. Murray
G and Colombo J. Ranolazine preserves and improves left ventricular ejection
fraction and autonomic measures when added to guideline-driven therapy in
chronic heart failure (2014) Heart International 9: 66-73. https://doi.org/10.5301/heartint.5000219
26. Murray
G and Colombo J. (r) Alpha Lipoic Acid is a safe, effective pharmacologic
therapy of chronic orthostatic hypotension associated with low sympathetic tone
(2019) Thieme Medical Publishers 333 Seventh Avenue, New York, USA. https://doi.org/10.1055/s-0038-1676957
27. Murray
G and Colombo J. Ranolazine therapy reduces non-ST-segment elevation myocardial
infarction and unstable angina in coronary disease patients with angina (2016)
Int J Angiology 25: 159-164. https://doi.org/10.1055/s-0036-1572364
28. Umetani
K, Singer DH, McCraty R and Atkinson M. Twenty-four hour time domain heart rate
variability and heart rate: relations to age and gender over nine decades
(1998) J Am Coll Cardiol 31: 593-601. https://doi.org/10.1016/s0735-1097(97)00554-8
29. Vinik
AI, Maser RE, Mitchell BD and Freeman R. Diabetic autonomic neuropathy (2003)
Diabetes Care 26: 1553-1579. https://doi.org/10.2337/diacare.26.5.1553
30. Maser
R, Mitchell B, Vinik AI and Freeman R. The association between cardiovascular
autonomic neuropathy and mortality in individuals with diabetes: a
meta-analysis (2003) Diabetes Care 26: 1895-1901. https://doi.org/10.2337/diacare.26.6.1895
31. DePace
NL, Mears JP, Yayac M and Colombo J. Cardiac autonomic testing and diagnosing
heart disease: A clinical perspective (2014) Heart International 9: 37-44. https://doi.org/10.5301/heartint.5000218
32. DePace
NL, Mears JP, Yayac M and Colombo J. Cardiac autonomic testing and treating
heart disease: A clinical perspective (2014) Heart International 9: 45-52. https://doi.org/10.5301/heartint.5000216
33. Bullinga
JR, Alharethi R, Schram MS, Bristow MR and Gilbert EM. Changes in heart rate
variability are correlated to hemodynamic improvement with chronic CARVEDILOL
therapy in heart failure (2005) J Card Fail 11: 693-699. https://doi.org/10.1016/j.cardfail.2005.06.435 34. Fatoni
C, Raffa S, Regoli F, Giraldi F, La Rovere MT, et al. Cardiac resynchronization
therapy improves heart rate profile and heart rate variability of patients with
moderate to severe heart failure (2005) J Am Coll Cardiol 46:1875-1882. https://doi.org/10.1016/j.jacc.2005.06.081
35. Fathizadeh
P, Shoemaker WC, Woo CCJ and Colombo J. Autonomic activity in trauma patients
based on variability of heart rate and respiratory rate (2004) Crit Care Med
32: 1300-1305. https://doi.org/10.1097/01.ccm.0000127776.78490.e4
36. Peng‐sheng
C, Chung‐chuan C, Tan AY, zhou S, Fishbein MC, et al. The mechanisms of atrial
fibrillation (2006) J Cardiovasc Electrophysiol 17: S2-S7. https://doi.org/10.1111/j.1540-8167.2006.00626.x
37. Copie
X, Lamaison D, Salvador M, Sadoul N, DaCosta A, et al. Heart rate variability
before ventricular arrhythmias in patients with coronary artery disease and an
implantable cardioverter defibrillator (2003) Ann Noninvasive Electrocardiol 8:
179-184. https://doi.org/10.1046/j.1542-474x.2003.08302.x
38. Alter
P, Grimm W, Vollrath A, Czerny F and Maisch B. Heart rate variability in
patients with cardiac hypertrophy-relation to left ventricular mass and
etiology (2006) Am Heart J 151: 829-836. https://doi.org/10.1016/j.ahj.2005.06.016
39. Debono
M and Cachia E. The impact of cardiovascular autonomic neuropathy in diabetes:
is it associated with left ventricular dysfunction? (2007) Auton Neurosci 132:
1-7. https://doi.org/10.1016/j.autneu.2006.11.003
40. Just
H. Peripheral adaptations in congestive heart failure: a review (1991) Am J Med
90: 23S-26S. 41. Nakamura
K, Matsumura K, Kobayashi S and Kaneko T. Sympathetic premotor neurons
mediating thermoregulatory functions (2005) Neurosci Res 51: 1-8. https://doi.org/10.1016/j.neures.2004.09.007
42. Manfrini
O, Morgagni G, Pizzi C, Fontana F and Bugiardini R. Changes in autonomic
nervous systemactivity: spontaneous versus balloon-induced myocardial ischaemia
(2004) Eur Heart J 25: 1502-1508. https://doi.org/10.1016/j.ehj.2004.03.019
43. Vinik
AI, Maser RE and Nakave AA. Diabetic cardiovascular autonomic nerve dysfunction
(2007) US Endocrine Dis 2: 66-74. 44. Clarke
B, Ewing D and Campbell I. Diabetic autonomic neuropath (1979) Diabetologia 17:
195-212. 45. Arora
RR, Bulgarelli RJ, Ghosh-Dastidar S and Colombo J. Autonomic mechanisms and
therapeutic implications of postural diabetic cardiovascular abnormalities
(2008) J Diabetes Sci Technol 2: 568-571. https://doi.org/10.1177/193229680800200416 Gary L Murray, The Heart and Vascular Institute, 7205 Wolf
River Blvd, Germantown, TN, 38138, USA, Tel: 901-507-3100, Fax: 901-507-3101,
E-mail: drglmurray@hotmail.com Murray LG and Colombo J. Routine measurements of
cardiac parasympathetic and sympathetic nervous systems assists in primary and
secondary risk stratification and management of cardiovascular clinic patients
(2019) Clinical Cardiol Cardiovascular Med 3: 27-33. Cardiac parasympathetic, Cardiac sympathetic, Coronary
diseases, Heart failure.Routine Measurements of Cardiac Parasympathetic and Sympathetic Nervous Systems Assists in Primary and Secondary Risk Stratification and Management of Cardiovascular Clinic Patients
Abstract
Objective: Full-Text
Introduction
Methods
Results
In
CHF, S is increased due to enhanced stimulatory input, increased adrenal
catecholamine output, as well as reduction of restraining influences, including
reduced vagal input, although beta-1 Adrenergic Receptors (AR) are down
regulated due to chronic stimulation. Beta- 2 AR, muscarinic and nicotinic
receptor function remains intact. Patients responding to Cardiac Resynchronization
Therapy (CRT) demonstrate improved P&S function, whereas non-responders
do not [20-23].
High
S-activity contributes to MACE through hemodynamic stress, coronary
vasoconstriction, cardiac electrical instability, endothelial dysfunction, and
LDL (Low Density
Lipoprotein) cholesterol oxidation. Alternatively, MACE acutely increases
S-activity and responsiveness. Therefore identifying high SB should help
predict the risk of developing MACE as well as diagnosing its presence.
Logically, normalizing SB will help to prevent MACE and reduce its mortality
and morbidity.
Orthostatic
hypotension occurs in 10-30% of the elderly, associated with significantly
increased mortality and morbidity. Resting P&S activity falls with aging.
Chronic disease accelerates the aging effect. The P-nervous system (comprised
primarily of the Vagus Nerve outside the brain) is more exposed, and therefore,
more susceptible to insult, including increased oxidative stress that occurs
with age. As a result, the P&S nervous systems become uncoupled, with
P-activity declining faster than S-activity. This imbalance leads to autonomic
dysfunction and ultimately autonomic neuropathy. A first sign of autonomic
dysfunction is orthostatic dysfunction, including SW which typically precedes
any decline in BP upon standing. SW (as in NOH) is a leading cause of orthostatic dysfunction.
We typically, pharmacologically, treat symptomatically with Midodrine,
Fluodrocortisone, Desmopressin, or occasionally with expensive Droxydopa
(Northera) or other drugs. (R)ALA, an over-the-counter powerful antioxidant
supplement (ALA is produced in the body and production declines with age),
treated the cause of NOH and NOI successfully in 66% of patients by increasing
S-responses with stand (relieving SW). Hopefully, treating the cause will slow
this diseases progression and reduce mortality and morbidity, as well as
treatment complications such as supine/sitting high BP and fluid overload.
P&S activity should be measured in all orthostatic patients without venous
stasis or medication-related orthostatic dysfunction. A concern of orthostatic
dysfunction is the possibility of low coronary perfusion. If coronary diastolic
BP is below 60 mmHg then the heart is hypo-perfused, as is the brain. As a
result, an adrenaline storm is released and systolic pressure is increased.
This increases cardiac demand and cardiac stress.
CAN is
defined as very weak, resting P-activity; regardless of resting S-activity. CAN
is a normal part of the aging process. It simply means that the typical elderly
person has a higher morbidity and especially mortality risk than the typical
younger person. However, this is not to dismiss CAN. As soon as CAN is
demonstrated, a full cardiac work-up is recommended, if for no other reason
than to establish a baseline. The other reason not to dismiss CAN is that it
should always be risk stratified. CAN is risk stratified by SB. Given that a
little more (resting) P-activity is cardio-protective, low-normal SB
(0.4Discussion
Conclusions
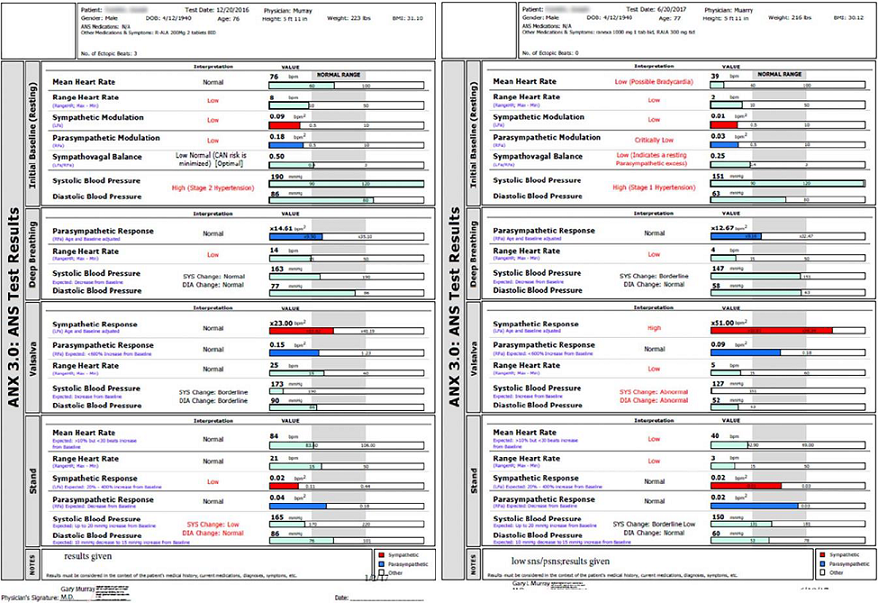
Future
Trials
We
have begun to investigate P&S abnormalities contributing to hypertension,
considering the question When is high blood pressure a symptom and better
treated as secondary? By 2021, worldwide, 1.5 billion or 1/3 of the worlds
population will be hypertensive. Currently, only 35% of patients are
clinic-controlled, 30% have masked-uncontrolled high BP (MUCH) Masked Uncontrolled
Hypertension, and after 1.5 yrs. of treatment, 25% return to uncontrolled
status. Often, we find high BP to be compensatory to decreases in BP upon
head-up posture, such as with SW in NOH or NOI. Apparently this is to help
maintain coronary and brain perfusion. This form of hypertension is often
relieved organically once SW and thereby orthostatic dysfunction is relieved
[45]. Another P&S finding associated with high BP that does not seem to
respond to standard therapy is associated with high S-activity secondary to
high P-activity. Typical therapy seems to exacerbate the high P-activity,
thereby forcing higher S-activity (since P-activity establishes the threshold
around which S-responds). As a result, BP becomes more labile or the patient
seems unresponsive.
We
plan a multicenter, randomized, prospective study: Management of Outpatients
using Sympathovagal Balance Trial (MOST) to compare P&S-guided therapy to
usual care (Figures 2,3). Our hypothesis generating findings include the
observation that SB>2.5 seems to be a better predictor of MACE (ACS, acute
CHF, VT/VF, cardiac death) when compared with reversible myocardial imaging defect(s)
or LVEF<0.34 in the same patients.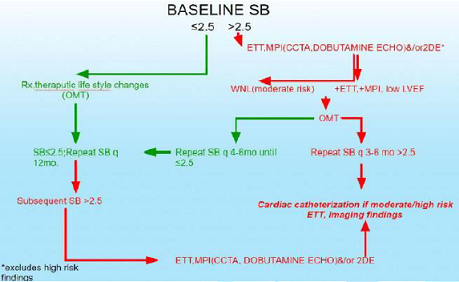
References
*Corresponding
author:
Citation:
Keywords