Introduction
Myxoma is the most common non-malignant primary cardiac tumor with an estimated incidence of 0.5 per million per year [1]. Before the introduction of invasive angiographic examination of the heart, cardiac myxomas were generally diagnosed at autopsy or rarely during cardiac surgery being done for other reasons [2-5]. The first cardiac myxoma was diagnosed by angiography in 1951 and the first surgical tumor removal was performed in 1954 [3, 6, 7]. Although 70% of cardiac myxoma cases were still being found at autopsy or during operation in the late 1950s and early 1960s, clinical cases diagnosed by cardiac angiography were gradually increasing [8, 9]. With the advent of echocardiographic techniques in 1960s, the diagnostic accuracy of cardiac myxomas was improved greatly. Echocardiography made the diagnosis of most cardiac myxoma cases possible during life, allowing for subsequent potentially curative surgical removal [10]. With the development of thoracic computed tomography (CT) in the early 1980s and magnetic resonance imaging (MRI) of cardiac structure in the late 1980s, these techniques have been applied to the detection of cardiac tumors, although echocardiography has remained the primary method of diagnosis of cardiac myxoma [11, 12].
As imaging utilization proliferated through the middle of 1990s [13-16] and on to today, cardiac as well as other neoplasms have been discovered. Many of these neoplasms were unexpected and/or asymptomatic. Asymptomatic or unexpected (incidental) neoplasms may possibly differ in their epidemiological, clinical and/ or pathological characteristics as well as their potential for safety of resection. In order to examine the use of modern imaging technologies, as applied to cardiac myxomas, we have assembled a single institution retrospective case series of cardiac myxomas discovered and resected from 2007-2013. This case series (Current Group) was evaluated for method of discovery, clinical characteristics, and epidemiology and then compared with previous large case series in the literature. With this study, we hope to see the evolution of diagnostic technologies as applied to the detection of atrial myxoma, and to determine whether the natural history or treatment effectiveness of atrial myxoma has changed.
Methods
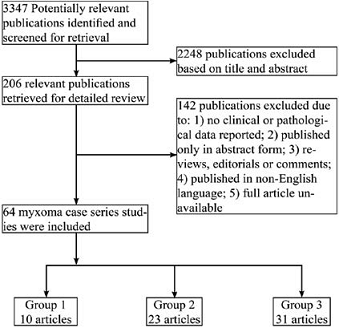
Following Institutional Review Board approval, all 28 patients with pathologically proven cardiac myxoma at Florida Hospital Orlando from April 2007 to August 2013 were studied. The patients medical records were reviewed, and data about clinical presentation, diagnostic methods and clinical course were collected. Following collection, data were de-identified for analysis. These dates were chosen because 2007 represented the beginning of electronic medical records use at Florida Hospital. Myxoma size was taken from pathology records. For comparative review, PubMed was searched from January 1985 to January 2014 by using the following keywords: “cardiac myxoma”, and studies published in English were included. The only inclusion criterion was a case series of cardiac myxoma with more than 10 cases. Articles were excluded if: 1) clinical and pathological data were not reported; 2) they were published only in abstract form; 3) they were reviews, editorials or comments; 4) they were published only in non-English language; 5) a full article was not available. Figure 1 displays the flow diagram of study selection. Our initial search yielded 3347 publications from January 1 1985 to December 31, 2013. After screening the titles and abstracts of all studies, 206 potentially relevant articles were selected for further screening. Eventually, 64 studies met the inclusion criterion and did not meet the exclusion criteria. In order to see changes over time in method of discovery, clinical characteristics, and epidemiology, these 64 studies were divided into three groups: Group 1: cases collected exclusively after 1995 (10 articles); Group 3: exclusively before 1995 (31 articles); and Group 2: those not belonging to group 1 or 3 (23 articles). We chose 1995 as the year for dividing the groups since imaging utilization started to increase exponentially around that time [13-16]. A full listing of all articles included in the study is contained in a supplemental table. Data from tables in each of the 64 articles were combined in the groups to prepare Table 1-4. Frequencies were simply summed. Means were weighted by frequency. When available, standard deviations were combined (after weighting) for statistical analysis [17]. Frequency tables were analyzed by Pearsons Chi-Square test of independence using SPSS (version 21). A Two-sided P value of less than 0.05 was considered statistically significant. Means were compared using one-way ANOVA followed by Tukeys pairwise comparison. Because many articles, especially in group 3, did not report variance information, statistical analysis of means data was limited to articles reporting variances.
Results
The Current Group contained 28 subjects, 20 female (71.5%) and 8 males (28.5%) with mean age 61.2 years (range, 41-86) (Table 1). Sixty one percent of subjects were between 51 and 70 years old. The mean age of the Current Group is statistically significantly higher than the pooled mean age of Groups 1 and 3 (One-way ANOVA p<0.001, Tukeys pairwise comparison p<0.05 compared to Groups 1 and 3). This finding may simply reflect the relatively small size of the Current Group. The mean ages of groups 1-3 were similar when all cases were included (mean age) or restricted to those studies reporting variance information (pooled mean age).
The frequency of females in the Current Group (71.5%) was slightly higher than previous reports, which ranged from 63.5 to 66.1% (Table 1). Of 28 subjects in the Current Group, 24 (85.7%) had left atrial myxomas and 4 (14.3%) had right atrial myxomas, which was similar to previous studies (X2=9.6, p=0.143) (Table 1). The average size of the tumor in the Current Group was 3.3 cm. The mean size of the Current Group was statistically significantly smaller than the pooled mean of group 3 (ANOVA, p<0.001, Tukey pairwise comparison p<0.05). The pooled mean size may not be reliable, however, due to sparse reporting of variance information in Group 3, particularly. The mean size of all cases shows a clear trend toward smaller tumors in more recent series (Table 1).
The major presenting symptoms and clinical features are summarized in table 2. In the Current Group, at time of diagnosis, 6 patients were asymptomatic (21.4%, all were in the left atrium), which is much higher than previous reports. The percentage of asymptomatic cases at time of diagnosis has significant increased over time (5.0% in group 1, 3.2% in group 2, 2.0 % in group 3, Table 2). Of the 6 asymptomatic cases in the Current Group, the largest myxoma was 3.1 cm in diameter and the mean size was 2.2 cm. Of the asymptomatic cases, 3 were first diagnosed by Figure 1: Flowchart of study search and selection. PubMed was searched for case series of cardiac myxoma from January 1, 1985 to December 31, 2013, and studies that met inclusion criteria were divided into three groups: Group 1: cases collected exclusively after 1995 (10 articles); Group 3: cases exclusively before 1995 (31 articles); and Group 2: those not belonging to group 1 or 3 (23 articles). transthoracic echocardiography (TTE), 2 by CT and 1 by MRI. The diagnosis of myxomas in these patients was made incidentally. In the symptomatic cases in the Current Group, dizziness/syncope was the most common symptom (28.6%), followed by dyspnea (25.0%) and chest pain (21.4%). Other cardiac presenting symptoms included palpitation (3 patients, 10.7%) and heart failure (1 patient, 3.6%). Three patients (10.7%) presented with stroke/TIA like symptoms. Another 3 patients presented with systemic symptoms: fatigue (2 patient, 7.1%) and fever (1 patient, 3.6%). As shown in table 2, the pattern of presenting symptoms over time has changed significantly (X2=235, p<0.001). Heart failure and embolic phenomena in particular have decreased over time (Table 2).
In the Current Group, 21 (75.0%) of myxomas were initially diagnosed by TTE (19 cases) or transesophageal echocardiogram (TEE) (2 cases), 5 (17.8%) by CT, 1 (3.6%) by MRI and 1 (3.6%) by coronary angiography (Table 3). TEE was performed in all cases to confirm the diagnosis before surgery. Coronary angiography was performed in patients with history of chest pain or those older than 45 years. The initial diagnostic imaging for establishing the diagnosis was reviewed and summarized in Table 3. Statistical analysis of data in Table 3 could not be performed due to sparse data for most of cells. Before 1995, most cases were diagnosed initially by M-mode echocardiography and TTE. After 1995, more than 97% of cases were first diagnosed by TTE or TEE. Before 1995, in 21.5% of cases, multiple imaging modalities were used to confirm the diagnosis and in 3.8% cases were diagnosed during operation, which was not reported again after 1995. New imaging techniques, such as CT and MRI, are used more frequently and are becoming more common as initial diagnostic tools to find myxoma. In contrast, cardiac angiography is rarely applied to diagnose myxoma since 1995.
All patients in the Current Group had surgery. One patient died during hospitalization. Average length of hospital stay was 11.2 days, and 4 patients were readmitted within 30 days. The most common perioperative morbidities were pleural effusion (42.8%) and pulmonary atelectasis (42.8%), followed by arrhythmia (25.0%) and anemia (25.0%). Twenty three patients received postoperative follow-up. The period of follow-up ranged from 5 months to 8 years. One patient died from congestive heart failure eight years after the procedure. No myxoma recurrence was observed in follow-up. Early perioperative mortality and long-term recurrence trends over different time periods in previous studies are summarized in Table 4. No significant difference was identified in either perioperative mortality (X2=3.7, p=0.155) or long-term recurrence (X2=0.7, p=0.754) between groups.
Discussion
Myxomas are the most common cardiac tumor. They usually occur in middle age and are more common in females. In the Current Group, the average age of the patients and the frequency of females is slightly higher than previous reports. The average size of the tumor is much smaller in the Current Group than previous reports and continues a trend of decreasing size over time. One possible reason could be the wide application of new imaging tools leading to early diagnosis of asymptomatic or mildly symptomatic cases. It has been reported in several studies that about 20% of cardiac myxomas are asymptomatic and they are usually smaller than 4 cm [18, 19].
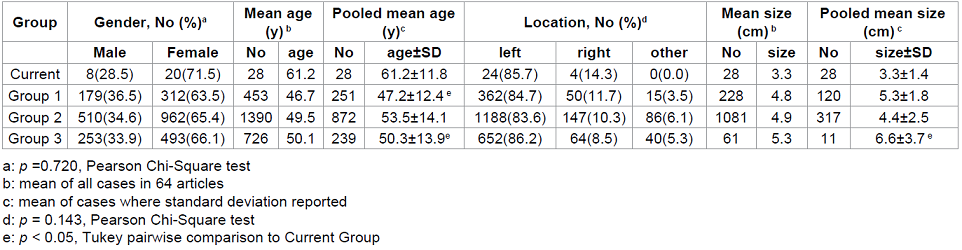
Table 1: Gender, age, location and size characteristics of cardiac myoxma by group.
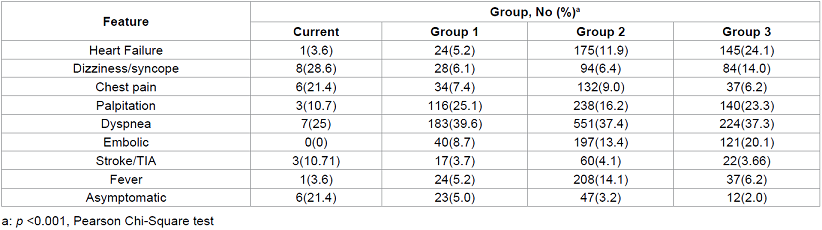
Table 2: Clinical features of cardiac myxoma by group.
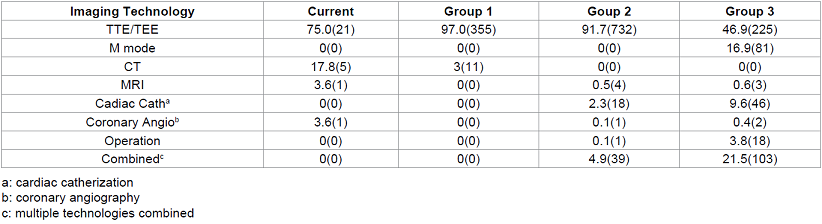
Table 3: Initial imaging technology for myxoma diagnosis by group.

Table 4: Mortality and Recurrence of cardiac myxoma.
In the Current Group, 6 cases (21.4%) were asymptomatic at diagnosis. The average tumor size in these asymptomatic cases was only 2.2 cm, which was much smaller than the average tumor size (Table 1). Of these 6 cases, half of them were initially found by new imaging technique CT and MRI rather than TTE, which are generally not considered as the first line diagnostic tool for myxoma diagnosis (Table 3).
Although in some cases, thrombus or other occupying lesions may be misdiagnosed as myxoma, both TTE and TEE are reported to have a high sensitivity for myxoma diagnosis, 95 and up to 100%, respectively [20]. TTE is non-invasive, and can measure the size and shape, locate the attachment site and identify the mobility of the tumor. In cases of patients with poor transthoracic echocardiographic window, TEE will provide imaging with better quality. TEE is particularly helpful to evaluate the posterior left atrial wall, atrial septum, and right atrium, which often are not well displayed on TTE, to exclude the possibility of biatrial multiple tumors [21]. Furthermore, TEE can provide more information for surgical resection regarding tumor size, location, mobility, and attachment [20, 22, 23] (Table 3).
CT and MRI are not the first diagnostic tools for myxoma at this time, although more and more cases are diagnosed by these technologies. If TTE and TEE provide limited tissue characterization, confident distinction between thrombi, benign and malignant tumors can usually be detected by CT or MRI [24]. For example, prolapse through the mitral valve orifice on CT is a reliable discriminatory finding indicating myxoma [25], while absence of both first pass and delayed contrast enhancement on MRI is suggestive of thrombus [26]. Cardiac CT is also useful to detect metastases in suspected malignancies especially when coupled with 18F-fluorodeoxyglucose (FDG) Positron Emission Tomography (PET). However, if a mass has a typical echocardiographic appearance and location of a left atrial myxoma, additional images with CT or MRI are unnecessary.
As shown in Table 3 and mentioned above, more and more cases are found by CT and MRI, even though they are not the first line of diagnostic tools for myxoma. One of the reasons is likely due to the significant advance of the techniques, which make them more sensitive to capture the intracardiac lesions. Another important reason is likely due to the overuse of diagnostic tools. A report by Iglehart indicated that, between 2000 and 2007, use of imaging studies grew faster than that of any other physician service in the Medicare population [27]. Another report by the influential group Americas Health Insurance Plans claimed that 20% to 50% of all “high-tech” imaging provides no useful information and may be unnecessary [28]. More specifically, CT order associated with common chest related symptom emergency room visits increased from 2.1% in 1997 to 1999 to 11.5% in 2005 to 2007, whereas the overall proportion of these visits associated with a clinically significant diagnosis decreased from 23.6% in 1997 to 1999 to 19.1% in 2005 to 2007 [15]. Studies about the overuse of TTE were also reported. Between 1999 and 2004, the use of TTE has increased by 10 percent each year in the United States, and 10- 15 percent of TTE studies performed do not meet appropriate use criteria [29, 30]. Does the incidental or accidental finding of myxoma by CT or MRI or the application of CT or MRI in myxoma management improve outcome? We do not have the final answer for this question. However, we found that the increased application of CT and MRI in myxoma diagnosis or management is not associated with decreased perioperative mortality or longterm recurrence.
There are several limitations for this study. First, the Current Group is of modest size, which may account for the age disparity from some previous studies. Second, the pooling of previously reported studies is inherently limited by publication and reporting biases and by lack of uniformity in data reporting.
Conclusion
In this study, the Current Group, when compared to contemporary historical groups, confirmed our suspicion that the proliferation of advanced imaging procedures during the mid1990s and beyond has resulted in the identification of cardiac myxomas that are smaller and have fewer traditional symptoms. Meanwhile, surgical mortality and recurrence rates for myxoma have remained low, leaving little opportunity for improvement despite dramatic improvements in surgical mortality for other cardiac conditions [31].
Acknowledgement
The authors wish to thank Dr. Julie Pepe for her assistance in statistics, Dr. Khalid Abusaada, Dr. Shengchuang Dai and Dr. Vladimir Pech for their suggestions and comments.
References
1. MacGowan SW, Sidhu P, Aherne T, Luke D, Wood AE, et al. Atrial myxoma: national incidence, diagnosis and surgical management. (1993) Ir J Med Sci 162: 223-226.
2. PRICHARD RW. Tumors of the heart; review of the subject and report of 150 cases. (1951) AMA Arch Pathol 51: 98-128.
3. Goldberg HP, Glenn F, Dotter CT, Steinberg I. Myxoma of the left atrium; diagnosis made during life with operative and post-mortem findings. (1952) Circulation 6: 762-767.
4. Differding JT, Gardner RE, Roe BB. Intracardiac myxomas with report of two unusual cases and successful removal. (1961) Circulation 23: 929-941.
5. McAllister HA Jr. Primary tumors and cysts of the heart and pericardium. (1979) Curr Probl Cardiol 4: 1-51.
6. Glover RP. Late results of mitral commissurotomy. (1955) In: Henry Ford Hospital international symposium on cardiovascular surgery: studies in physiology, diagnosis and techniques: proceedings of the symposium, Lam CR (edtr), Saunders, Philadelphia, USA.
7. Reynen K. Cardiac myxomas. (1995) N Engl J Med 333: 1610-1617.
8. Bulkley BH, Hutchins GM. Atrial myxomas: a fifty year review. (1979) Am Heart J 97: 639-643.
9. St John Sutton MG, Mercier LA, Giuliani ER and Lie JT. Atrial myxomas: a review of clinical experience in 40 patients (1980) Mayo Clinic proceedings 55:371-6.
10. Schattenberg TT. Echocardiographic diagnosis of left atrial myxoma. (1968) Mayo Clin Proc 43: 620-627.
11. Huggins TJ, Huggins MJ, Schnapf DJ, Brott WH, Sinnott RC, et al. Left atrial myxoma: computed tomography as a diagnostic modality. (1980) J Comput Assist Tomogr 4: 253-255.
12. Pflugfelder PW, Wisenberg G, Boughner DR. Detection of atrial myxoma by magnetic resonance imaging. (1985) Am J Cardiol 55: 242-243.
13. Lang K, Huang H, Lee DW, Federico V, Menzin J. National trends in advanced outpatient diagnostic imaging utilization: an analysis of the medical
expenditure panel survey, 2000-2009. (2013) BMC Med Imaging 13: 40.
14. Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010 Jama 307:2400-9.
15. Coco AS and OGurek DT. Increased emergency department computed tomography use for common chest symptoms without clear patient benefits (2012) Journal of the American Board of Family Medicine : JABFM 25:33-41.
16. Semin S, Demiral Y and Dicle O. Trends in diagnostic imaging utilization in a university hospital in urkey (2006) International journal of technology assessment in health care 22:532-6.
17. Arsham H. Pooling the Means, and Variances 2015.
18. Goswami KC1, Shrivastava S, Bahl VK, Saxena A, Manchanda SC, et al. Cardiac myxomas: clinical and echocardiographic profile. (1998) Int J Cardiol 63: 251-259.
19. Grebenc ML, Rosado-de-Christenson ML, Green CE, Burke AP and Galvin JR. Cardiac myxoma: imaging features in 83 patients (2002) Radiographics: a review publication of the Radiological Society of North America, Inc 22:673-89.
20. Engberding R, Daniel WG, Erbel R, Kasper W, Lestuzzi C, et al. Diagnosis of heart tumours by transoesophageal echocardiography: a multicentre study in 154 patients. European Cooperative Study Group (1993) European heart journal 14:1223-8.
21. Lad VS, Jain J, Agarwala S, Sinha VK, Khandekar JV, et al. Right atrial transseptal approach for left atrial myxomas--nine-year experience. (2006) Heart Lung Circ 15: 38-43.
22. Obeid AI, Marvasti M, Parker F, Rosenberg J. Comparison of transthoracic and transesophageal echocardiography in diagnosis of left atrial myxoma. (1989) Am J Cardiol 63: 1006-1008.
23. Ha JW, Kang WC, Chung N, Chang BC, Rim SJ, et al. Echocardiographic and morphologic characteristics of left atrial myxoma and their relation to systemic embolism (1999) The American journal of cardiology 83:1579-82.
24. Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. (2005) Radiographics 25: 1255-1276.
25. Scheffel H, Baumueller S, Stolzmann P, Leschka S, Plass A, et al. Atrial myxomas and thrombi: comparison of imaging features on CT. (2009) AJR Am J Roentgenol 192: 639-645.
26. ODonnell DH, Abbara S, Chaithiraphan V, Yared K, Killeen RP, et al. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. (2009) AJR Am J Roentgenol 193: 377-387.
27. Iglehart JK. Health insurers and medical-imaging policy--a work in progress. (2009) N Engl J Med 360: 1030-1037.
28. Rao VM, Levin DC. The overuse of diagnostic imaging and the Choosing Wisely initiative. (2012) Ann Intern Med 157: 574-576.
29. Pearlman AS, Ryan T, Picard MH, Douglas PS. Evolving trends in the use of echocardiography: a study of Medicare beneficiaries. (2007) J Am Coll Cardiol 49: 2283-2291.
30. Ward RP, Mansour IN, Lemieux N, Gera N, Mehta R, et al. Prospective evaluation of the clinical application of the American College of Cardiology Foundation/American Society of Echocardiography Appropriateness Criteria for transthoracic echocardiography (2008) JACC Cardiovascular imaging 1: 663-71.
31. Ghali WA, Ash AS, Hall RE, Moskowitz MA. Statewide quality improvement initiatives and mortality after cardiac surgery. (1997) JAMA 277: 379-382.
*Corresponding author:
Junhong Gui, 2501 North Orange Avenue, Suite 235, Orlando, FL 32804, United States; Tel: 407 303 7270; Fax: 407 303 2553, E-mail: Junhong.Gui.MD@flhosp.org
Citation:
Gui J, Maqsood A, Khadka S, Rodriguez K, Everett G (2015) New Trend of Cardiac Myxoma - Case Series and Systematic Review. CCCM 101: 1-5.
Keywords
Cardiac myxoma, Chi-Square test, ANOVA test.


 PDF
PDF