Introduction
Diabetics
have a two-fold increased risk of Sudden
Cardiac Death (SCD), the most common cause of death in adult diabetics.
Subgroup analyses have not explained this adequately [1]. Diabetic Autonomic
Neuropathy (DAN) [2], carries a 53% 5yr. mortality, half of the deaths sudden
[3]. DAN can progress to Cardiovascular
Autonomic Neuropathy (CAN) in approximately 65% of patients with aging and
diabetes duration [4]; CAN, critically low Parasympathetic tone (P), increased
SCD in the Framingham Study [5]. Hyperglycemic- oxidative stress causes
dysautonomia [6-8]. We hypothesized (r)-ALA, a natural, potent anti-oxidant,
might reduce SCD in Type 2 Diabetics (DMII) with dysautonomias. We have shown
previously (r)-ALA improves autonomics in Hypertension (HTN) [9] as well as
Neurogenic Orthostatic Hypotension (NOH) [10].
Methods
In
2006, 133 consecutive DMII referrals for cardiovascular evaluation underwent P
and S testing via ANX 3.0 Autonomic Monitoring (P&S Monitoring, Physio PS,
Inc., Atlanta, GA). P&S were computed simultaneously and independently by
concurrent, continuous time-frequency analysis of Respiratory
Activity (RA) and Heart Rate Variability (HRV), as we detailed previously
[11-17]. P&S srenormally; sitting LFa and RFa=0.5 to 10.0 bpm2;
SB is age dependent=0.4 to 1.0 for geriatrics; stand LFa is ≥ 10% increase with
respect to (wrt) sit; stand RFa is a decrease wrt sit. High SB is defined
as>2.5, as established in our 483 patient study [18]. High SB and CAN define
a high risk of mortality, Acute Coronary Syndromes (ACS), CHF, and Ventricular
Tachycardia/Fibrillation (VT/VF) alone or as a composite endpoint [18].
In
the 83 (r)-ALA patients (Group 1), P&S were recorded 2-3 mo. afterwards
until maintenance dosage, then yearly. Non-(r)ALA patients (Group 2, refused
(r)-ALA) were tested yearly. Exclusion criteria were (1) arrhythmia precluding
HRV measurement, and (2) cancer within 5 yrs. The inclusion criterion was DM II
with any abnormality of P or S. Informed consent was obtained for this open-label,
un-blinded study. The cause of SD was determined from hospital records or death
certificates. Out of hospital SCD was defined as pulse less SD of cardiac
origin.
Group
1 patients were subcategorized: survivors, Group AA; non-survivors Group AD.
Group 2 (Controls): survivors, Group NA; non-survivors, Group ND. All patients
took aspirin. All patients had Cardiovascular Autonomic Reflex Test (CART) w/o
isometric grip (grip has only 25% sensitivity for CAN) [19]. DAN was defined as
any abnormality of S or P, or high SB. CAN was defined as P<0.10bpm2,
or 2 abnormalities of CARTs. Median follow-up was 5 yrs. Mean age was 66 y/o.
There were 83 males, 50 females. Upon referral, rhythm assessment (Holters ±
event monitors) were performed if clinically indicated: Groups AA 60%, AD
57.1%, NA 60.7%, ND 31.8%.
The
abbreviations are: Δ, change from initial to final; A1C, glucose form
hemoglobin; (r) ALA ((r)Alpha-Lipoic Acid) (the r-isomer functional in humans);
BMI (Body Mass Index); Bx (Baseline); CAN; DAN; dBP (Diastolic Blood Pressure);
HL (Hyperlipidemia); HR (Heart Rate); Init (Initial); LFa ((Low Frequency
area)=S)); LVEF (Left Ventricular Ejection Fraction); mg (milligrams); N
(number); Nml (normal); ns (not significant); P (Parasympathetic tone); PE (Parasympathetic
Excess); QTc (corrected QT); RFa ((Respiratory Frequency area)=P)); S
(Sympathetic tone); SB (Sympathovagal Balance); sBP (systolic BP); SW
(Sympathetic Withdrawal). Given the size of the cohort, statistical
significance is p<0.100. Statistical significance was determined with either
a two-tailed, student T-test or a Pearson correlation.
Results
25% of (r)-ALA patients experienced SCD vs. 44% non-(r)-ALA patients, a 43% Relative Risk Reduction (RRR, p=0.0076 [Figure 1]), altering the natural history of DAN [3].

Figure 1: Sudden Death Mortality risk of a Diabetic type 2 cohort from a south-central USA cardiology practice. (r)ALA (blue curve) reduced this cohort’s relative risk ratio (RRR) by 43% (p=0.0076) as compared to controls (brown curve).
Demographics
Table 1 Survivor
demographics Group AA had significantly more males and higher final A1C; their
initial LVEF was insignificantly lower, factors not favoring survival [20-24];
tending to favor survival were insignificantly fewer with CAD (although all AA
and NA patients were vascularized with normal stress tests), less Chronic
Kidney Disease (CKD); and significantly more Angiotensin blocker therapy
(ACEI or ARB, p<0.100) [20,25]. 11% more (r)-ALA patents required insulin.
Control Group NA had significantly more females and lower final A1C; there were
insignificantly higher initial LVEFs and insignificantly more patients on
Empagliflozin, Liraglutid, and Metformin, tending to favor survival [26-29].
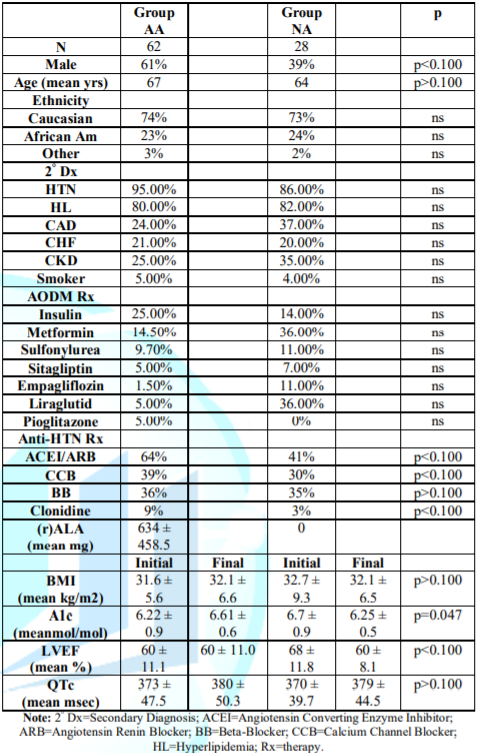
Table 1: Survivor Patient
Demographics.
Table 2 Non-Survivors.
Group AD had significantly more males and higher A1C; there were
insignificantly higher final BMI [24], lower LVEFs, more CHF, and less
Metformin use, all tending unfavorably regarding survival. But 9% more took
ACEI/ARBs (p<0.100). Control Group ND was 4 years older (p>0.100); QTc
had no significance on SD, as SD increases when QTc is >450ms in males or
>470ms in females [30]. Insignificantly more Group ND African Americans
tends to favor SD [31]. CAD causes most adult SDs [24]. Although more SD
patients had CAD vs. survivors, CAD prevalence was insignificantly different in
Groups AD, ND.
Group AA vs.
Group ND: Improved
Group AA survival occurred despite Group ND having a normal final BMI
(p=0.067), less HTN (p=0.021), greater use of Empagliflozin (p<0.100),
Metformin (p<0.100), lower final A1C (p=0.034), and fewer males
(p<0.100), all favoring less SCD in Group ND. DMII attenuates gender
differences in SD [22]. Group ND was 3 yrs. Older (p=0.067) with more CAD
(p<0.100); all were revascularized (normal myocardial perfusion stress
tests). Fewer in Group AA took insulin (p<0.100). Initially, Group AA had
18.4% VT (1sustained) vs. 14.3% non-sustained in Group ND, p=0.3559.
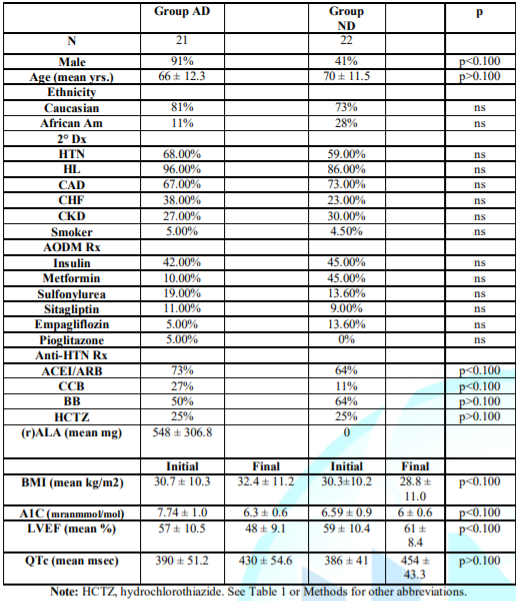
Table 2: Non-Survivor Patient
Demographics (Sudden Death Patients).
Group NA vs.
Group AD: NA
patients were 2 yrs. Younger (p=0.081); more hypertensive (p=0.086); had
greater use of Empagliflozin (p<0.100), Metformin (p<0.100), Liruglutid
(p<0.100), higher final LVEFs (60% vs. 48%, p<0.100), fewer males
(p<0.100), and less CAD (p<0.100; revascularized with normal stress
tests), mostly favoring survival. Fewer in Group NA took insulin (p<0.100).
Initially, Group NA had 0% non-sustained VT vs. 16.7% in Group AD, p=0.1661.
Autonomic
Measures: Table 3:
Survivors and SCD patients initial to final autonomic Measures. Mean Bx LFa,
decreased in survivors (p=0.045), increasing in SCD (p=0.039). Bx RFa,
increased in 55/90 patients (60%), by a mean 12.5% in survivors and severely
decreased in 29/43 (67%) non-survivors, mean -59.5%, (p<0.0001). SB
increased 17.6% in survivors, but had a greater increase in SCD to >2.5:
+29.5% (p=0.064).
Non-Survivors
demonstrated a more abnormal final alpha-S-response standing, SW (-24.4% vs.
-13.8% [p=0.066]), indicating greater Bar receptor Reflex dysfunction, which
increases SCD risk. PE upon standing developed more significantly in survivors
(+65%) vs. SCD (+29%) because initial to final standing RFa increased in
survivors vs. decreasing in SCD (p=0.022). In parallel, SCD patients
experienced a dramatic 59.5% decrease in resting P in addition to SW. All P-
and S- final values were lower in SCD, the lowest being resting P. Since
HRV=S+P, HRV was lower in SCD (p<0.0001) mainly due to lower P.
Survivors
Group-AA,
Survivors with (r)-ALA: (Table 4) A1C increased (increasing oxidative
stress, p=0.047), inversely proportional to (r)-ALA dosage (p=0.071); but
resting RFa increased proportionally (p=0.014). Average resting Bx LFa
increased (p=0.095) as did resting Bx RFa (p=0.070). HRV increased. The mean
initial standing response was SW. At final testing, 4 patients’ SW were
relieved (p=0.097); consequently, BRS improved. One more patient demonstrated
PE (p=0.098) (standing RFa increased) proportional to (r)-ALA dosage.
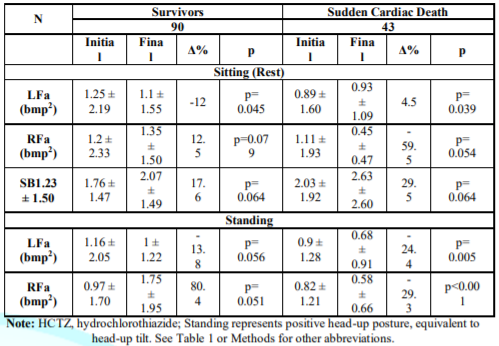
Table 3: Comparison between Survivors and Sudden Cardiac Death patients, Mean P&S Measures. See Methods for parameters’ normal ranges.

Table 4: Mean P&S
measures for DM II Survivors on (r)ALA (GroupAA).
Group-NA, Survivors
without (r)-ALA:
(Table 5) Similar to Group-AA, the
average initial P&S levels are normal, and given their age, SB is high (but
lower than Group AA and not >2.5). Contrary to Group AA, final BxLFa
decreased (p=0.075), as did BxRFa (and HRV). SB increased (p=0.088). Standing,
Group-NA initially demonstrated normal P- and slightly low S-responses.
Individually, 57.1% demonstrated SW. Of these, 81.3% demonstrated PE. At final
testing, 2 patients’ SW were relieved; 5 relieved PE, different from the Group
AA patients (p<0.027).
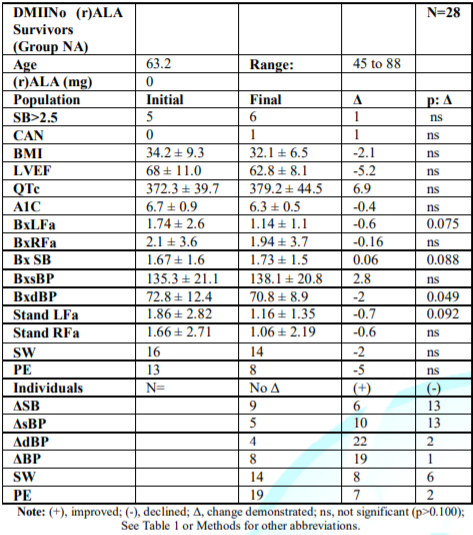
Table 5: Mean P&S measures
for DM II Survivors not on (r)ALA (Group NA), the control group.
Survivors’
Mortality Risk: 13%
Group AA patients demonstrated CAN initially, improving to 8.1%, proportional
to (r)-ALA dose (p=0.004). Group AA was the only Group that increased resting
BxRFa, (Table 4). Group-AA’s final RFa increased 36.2%, correlating with the
dose of (r)-ALA (p=0.014). Group AA’s increase in resting BxLFa (Table 4) was
mitigated by the increase in resting BxRFa, so the SB change was insignificant.
Group NA had no CAN initially; increasing to 3.6%. This group’s average resting
BxLFa decreased (34.5%); BxRFa fell 7.6%. SB (the average of 4 sec. ratios, not
the ratio of these reported averages) significantly increased 3.6% (p=0.088),
increasing MACE risk. In Tables 4 and 5, Group AA’s BxLFa and BxRFa were
initially lower than Group NA’s (p<0.100), indicating lower HRV. Group AA
increased both, decreasing mortality risk (Table 4). Group NA decreased both
BxLFa (Table 5) (p=0.075) and BxRFa (p=ns), indicating an accelerated
progression towards increased mortality risk (decreased HRV).
Non-Survivors
Group AD,
Non-Survivors with (r)-ALA: (Table 6) Initial P&S levels are below normal
and lowest of all Groups (lowest HRV). Given their age, SB is high (but not
>2.5). Final LFa increased (p=0.047); RFa decreased (p=0.098); and SB
increased to 2.72. Resting P protects against VT/VF and silent ischemia
[21,32-36]; seven progressed to CAN (p=0.080), not surprising since initial
BxRFa was so severely depressed. Group AD was beyond help. Standing, 57% of
Group AD initially demonstrated PE; 33% ended with PE (p=0.061) and 57% ended
with SW (p=0.037) indicative of BRS dysfunction (increases SCD). Finally, Group
AD’s, average stand LFa was SW. These Sympathetic results are significantly
similar to Group AA (p=0.061). However, the P-responses, are different
(p=0.185).
Group ND,
Non-Survivors without (r)-ALA: (Table 7) Initial resting BxLFa, resting
BxRFa, were normal; SB high for age (but not >2.5 Final BxLFa decreased,
p=0.100; BxRFa severely decreased, p=0.020. Two more patients (67%) developed
CAN (p=0.020) in spite of initially good BxRFa. Group ND’s initial standing P
was normal, but S showed SW. Final average S-stand remained SW; P barely
normalized. The P-responses as compared with the Group-AA are different
(p=0.106).
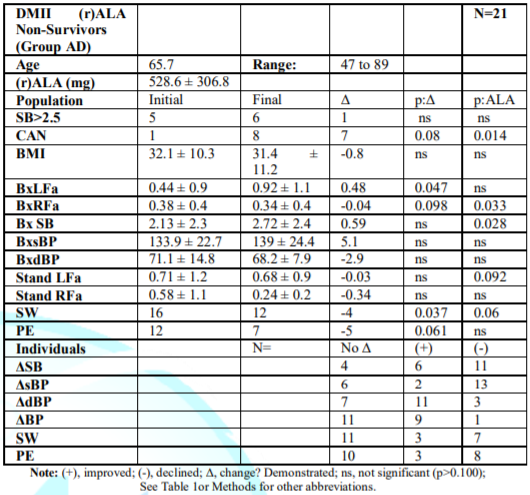
Table 6: Mean P&S
measures for DM II Non-Survivors on (r)ALA (Group AD).
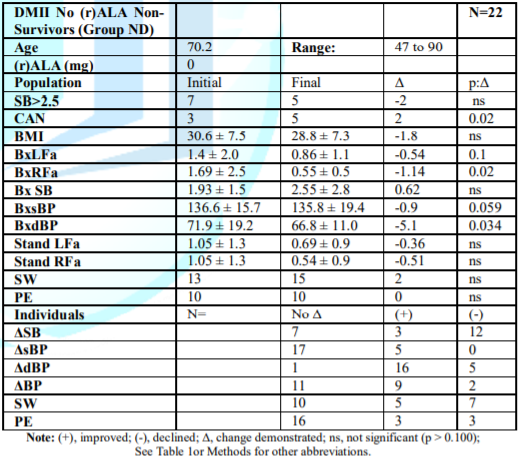
Table 7: Mean P&S
measures for DM II Non-Survivors not on (r)ALA (Group ND).
Mortality Risk: Resting BxRFa
decreased in both Groups (Tables 6&7): 10.5%, Group AD and 67.5%, Group ND
(p=0.033); a higher risk of developing CAN. Final SB was >2.5 in both, which
we have shown increases MACE 700% [18]. SB greater than 2.5 with CAN is
particularly deadly in both Groups, and final average standing response was SW
(impaired BRS), increasing SCD as well. BxLFa increased in Group AD (Table 6)
by 109.1% vs. decreasing 38.6% in Group ND (Table 7, p=0.100), causing
increased SB in Group AD.
In
Group ND, despite the decrease in S, the severe decrease in resting BxRFa
increased SB anyway. Two more patients had CAN. Non-survivors’ (r)ALA preserved
their severely lowest P and S (LOWEST HRV) even in death. Group ND’s final
BxLFa and BxRFa fell severely to the 2nd lowest among all Groups.
CAN and high SB were most frequent in Groups AD and ND.
Traditional
Standards Comparison: Comparing
the gold standard of CARTs, without isometric hand-grip, to any abnormality of
P&S Monitoring for diagnosing DAN or CAN, CARTs’ sensitivity was 48.2% of
Group 1 and 30.0% of Group 2 patients; an overall unsatisfactory sensitivity of
41.4%.
Discussion
Administration
of (r)ALA resulted in a 43% RRR of SCD, rather than the demographics that may
have favored survival in Controls. Rapid separation of the SCD curves (Figure
1) strongly implies treatment effect. Lower initial HRV, Group 1 vs. Group 2,
p<0.0001, predicted SCD: AA 1.83 vs. AD 0.82, p=0.0171; NA 4.14 vs. ND 3.09,
p=0.0051. More initial CAN ((rALA 10.8% vs. Controls 6%, p=0.0013) and initial
BRS dysfunction ((r)ALA 63.9% vs. Controls 58%, p=0.0044) predicted SCD better
than recorded VT. (r)ALA preserved P and S vs. Controls. Those with the lowest
P&S (HRV) died. Reduced HRV is a common thread in SCD Only Group AA
demonstrated an increase in final, resting P (and HRV); P reduces VT/VF and
silent ischemia [21,32-36], increasing 36.2% vs. a 7.6% decrease for Group NA,
a 10.5% decrease for Group AD, and a 67.5% decrease for Group ND.
The
progressive increase in the decline of resting P indicated mortality, from the
lowest decline in resting P in Group NA, to the next greater decline in Group
AD, to those with the greatest decline, Group ND (p<0.001). Changes in P
were proportional to (r)ALA dose. These trends are not found in the other
physiologic measures: BMI, LVEF, and QTc; and only different between the
survivors’ A1Cs (Group AA vs. Group NA, p=0.034). Since SW and PE can cause
both NOH and systemic HTN [9,10]. DMII patients not on (r)ALA might experience
orthostasis, or labile HTN. HTN could be secondary (neurogenic), and is over
twice as well controlled treating the primary SW ± PE [9] than treating the BP
per se. (r)ALA preserved P and S, especially P, in survivors and non-survivors.
(r)ALA is a natural, powerful thiol antioxidant. (r)ALA restores and recycles
vitamins A,C,E and glutathione [9,10,34].
It
improves hyperglycemia, endothelial dysfunction, nitric oxide levels
(protective against VT/VF, silent ischemia [37-40]), reduces nuclear kappa B,
and is essential for certain mitochondrial oxidative enzymes. (r)ALA prevents
diabetic-induced reduction of the afferent limb function of the baroreceptor
reflex (BR) [41], reducing MACE. SW, found in 50% to 74% of patients,
failed to correct in 88% of Group NA and all SCD patients. SW disappeared
substantially only in Group AA, 59.7% reduced to 53.2%, p=0.097, decreasing SCD
risk. The other most common, and most important, P&S finding was low
resting P in 56% to 81% of patients, improving only in Group AA (initial 56%,
final 9%; p=0.070), vs. Group NA (initial 29%, final 43%; p=0.098), and
worsening most severely in Group ND patients, a 67% reduction in RFa vs. 10.5%
reduction in Group AD (p=0.020).
CAN
decreased 37.5% in Group AA vs. an increase of 67% in Group ND. 29% of Group AD
had high SB vs. 50% in Group ND (p=0.037). More CAN in Group 2 increased
mortality; high SB increased mortality risk in Group 1. Group 1’s autonomic
profiles generally stabilized or improved (HRV); Group 2’s deteriorated,
especially a 59.5% decrease in resting P, reducing Group 2’s ability to combat
VT/VF, silent ischemia, and life stresses. Standard deviations decreased over time,
with the most decreases correlating with the (r)ALA dosage. The pleotropic
effects of (r)ALA likely contributed to SCD reduction. Increased nitric oxide
improves P&S, endothelial dysfunction, protects against VT/VF and silent
ischemia [37-40]. Decreased nitric oxide levels prolong QTc [37]. Improved
mitochondrial function should reduce SCD also [42]. Asymptomatic SW (BR
dysfunction) was the most common presentation of DAN. Approximately 90% of
patients had HTN, presumed to be essential (primary), not possibly secondary to
DAN. Ultimately, CAN with, or without, dangerously high SB can develop while
under our care. How simple it is to diagnose and treat dysautonomia early; how
tragic it may be not to.
Limitations
This
was not a double-blind, randomized, placebo-controlled study. Also, in autopsy
studies, not all SDs are cardiac.
Conclusions
(r)ALA
given to geriatric DMII patients with even minimal dysautonomia reduced SCD
43%, p=0.0076, due to improved P&S, increasing HRV, probably assisted by
its pleotropic effects, altering DAN’s natural history. Since CARTs detected
only 41% of dysautonomia, non-CARTs screening of DMII is recommended. The ANX
3.0 Autonomic Monitor provides the only independent measures of P&S. It is
our preferred assessment, allowing (r)ALA titration. If CARTs is done and
normal, non-diagnostic, or not done, we recommend empiric (r)-ALA 600mg/d.
References
1. Aune
D, Schlesinger S, Norat T and Riboli E. Diabetes mellitus and the risk of
sudden cardiac death: a systematic review and meta-analysis of prospective
studies (2018) Nutr Metab Cardiovasc Dis 28: 543-556. https://doi.org/10.1016/j.numecd.2018.02.011
2. Vinik
A, Mitchell B, Maser R and Freeman R. Diabetic Autonomic Neuropathy (2003)
Diabetes Care 26: 1553-1579. https://doi.org/10.2337/diacare.26.5.1553
3. Ewing
D, Campbell I and Clarke B. The natural history of diabetic autonomic neuropathy
(1980) Q J Med Winter 49: 95-108.
4. Spallone
V, Ziegler D, Freeman R, Bernardi L, Frontoni S, et al. Cardiovascular
autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and
management (2011) Diabetes Metab Res Rev 27: 639-653. https://doi.org/10.1002/dmrr.1239
5. Tsuji
H, Larson M, Venditti F, Manders E, Evans J, et al. Impact of reduced heart
rate variability on risk of cardiac events: The Framingham Heart Study (1996)
Circulation 94: 2850-2855. https://doi.org/10.1161/01.cir.94.11.2850
6. Rolo
A and Palmeira C. Diabetes and mitochondrial function: role of hyperglycemia and
oxidative stress (2006) Toxicol Appl Pharmacol 212: 167-178. https://doi.org/10.1016/j.taap.2006.01.003
7. Hussain
N and Adrian T. Diabetic Neuropathy: Update on pathophysiological mechanism and
the possible involvement of glutamate pathways (2017) Curr Diabetes Rev 13:
488-497. https://doi.org/10.2174/1573399812666160624122605
8. Yorek
M. The role of oxidative stress in diabetic vascular and neural disease (2003)
Free Radic Res 37: 471-480.
9. Murray
G and Colombo J. The feasibility of blood pressure control with
autonomic-assisted hypertension therapy versus JNC 8 therapy (2020) Clinical
Cardiol Cardiovascular Med 4: 1-5. https://doi.org/10.31579/2641-0419/055
10. Murray
G and Colombo J. (r)Alpha lipoic acid is a safe, effective pharmacologic
therapy of chronic orthostatic hypotension associated with low sympathetic tone
(2019) Int J Angiol 28: 188-193. https://doi.org/10.31579/2641-0419/055
11. Aysin
B, Colombo J and Aysin E. Comparison of HRV analysis methods during orthostatic
challenge: HRV with respiration or without? 2007 29th Int Conf IEEE
EMBS Lyon, France.
https://doi.org/10.1109/iembs.2007.4353474
12. Akselrod
S, Gordon D, Ubel F, Shannon D, Berger A, et al. Power spectrum analysis of
heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular
control (1981) Sci 213: 220-222.
https://doi.org/10.1126/science.6166045
13. Akselrod S, Gordon D, Madwed J, Snidman N, Shannon D, et al. Hemodynamic regulation: Investigation by spectral analysis (1985) Am J Physiol 249: H867-H873. https://doi.org/10.1126/science.6166045
14. Akselrod S, Eliash S, Oz O and Cohen S. Hemodynamic regulation in SHR: investigation by spectral analysis (1987) Am J Physiol 253: H176-H183. https://doi.org/10.1152/ajpheart.1987.253.1.h176
15. Akselrod S. Spectral analysis of fluctuations in cardiovascular parameters: a quantitative tool for the investigation of autonomic control. Trends Pharmacol Sci 9: 6-9. https://doi.org/10.1016/0165-6147(88)90230-1
16. Colombo
J, Arora R, DePace N and Vinik A. Clinical Autonomic Dysfunction: Measurement,
Indications, Therapies, and Outcomes (2014) Springer Science, USA.
17. Bloomfield
DM, Kaufman ES, Bigger JT Jr, Fleiss J, Rolnitzky L, et al. Passive head-up
tilt and actively standing up produce similar overall changes in autonomic balance
(1997) Am Heart J 134: 316-320. https://doi.org/10.1016/s0002-8703(97)70140-6
18. Murray
G and Colombo J. Routine measurements of cardiac parasympathetic and
sympathetic nervous systems assists in primary and secondary risk
stratification and management of cardiovascular clinic patients (2019) Clinical
Cardiovascular Med 3: 27-33. https://doi.org/10.33805/2639.6807.122
19. Korei A, Kempler M, Istenes I, Vagi D, Putz Z, et al. Why not use the handgrip test in the assessment of cardiovascular autonomic neuropathy among patients with diabetes mellitus? (2017) Curr Vasc Pharmacol 15: 66-73. https://doi.org/10.2174/1570161114666160822154351
20. Kannel
W and Schatzkin A. Sudden death: lessons from subsets in population studies
(1985) J Am CollCardiol 5: 141B-149B. https://doi.org/10.1016/s0735-1097(85)80545-3
21. Umetani
K, Singer DH, McCraty R, and Atkinson M. Twenty-four hour time domain heart
rate variability and heart rate: Relations to age and gender over nine decades
(1998) JACC 31: 593- 601.
https://doi.org/10.1016/s0735-1097(97)00554-8
22. Kucharska-Newton
A, Couper D, Pankow J, Prineas R, Rea T, et al. Diabetes and the risk of sudden
cardiac death, the Atherosclerosis Risk in Communities Study (2010) Acta
Diabetol 47: 161-168.
23. Patel
R, Moorthy M, Chiuve S, Pradhan A, Cook N, et al. Hemoglobin A1c levels and
risk of sudden cardiac death: A nested case-control study (2017) Heart Rhythm 14:
72-78.
https://doi.org/10.1016/j.hrthm.2016.08.044
24. Kuriachan
V, Sumner G and Mitchell L. Sudden cardiac death (2015) Curr Probl Cardiol 40:
133-200.
25. Aljaroudi W, Refaat M, HabibR Al-Shaar L, Singh M, Gutmann R, et.al. Effect of angiotensin-converting enzyme inhibitors and receptor blockers on appropriate implantable cardiac defibrillator shock in patients with severe systolic heart failure (from the GRADE Multicenter Study (2015) Am J Cardiol 1: 924-931. https://doi.org/10.3410/f.725363020.793512706
26. Suttar N, McLaren J, Kristensen S, Priess D and McMurray J. SGLT2 inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what were the likely mechanisms? Diabetologia (2016) 59: 1333-13339. https://doi.org/10.1007/s00125-016-3956-x
https://doi.org/10.1001/archinternmed.2010.409
28. Simard P, Presse N, Rov L, Dorais M, White-Guay B, et al. Association between metformin adherence and all-cause mortality among new users of metformin: A nested case-control study (2018) Ann Pharmacother 52: 305-313. https://doi.org/10.1177/1060028017743517
29. Costa
E, Goncalves A, Areas M and Morgabel R. Effects of metformin on QT and QTc
interval dispersion of diabetic rats (2008) Arg Bras Cardiol 90: 232-238.
30. Straus
S, Kors J, De Bruin M, van der Hooft C, Hofman A, et al. Prolonged QTc interval
and risk of sudden cardiac death in a population of older adults (2006) J Am
Coll Cardiol 47: 362-367. https://doi.org/10.1016/j.jacc.2005.08.067
31. Reinier
K, Rusinaru C and Chugh S. Race, ethnicity, and the risk of sudden death (2019)
Trends Cardiovasc Med 29: 120-126.
32. Curtis
BM and O’Keefe JH. Autonomic tone as a cardiovascular risk factor: The dangers
of chronic fight or flight (2002) Mayo Clin Proc 77: 45-54. https://doi.org/10.4065/77.1.45
33. Kalla
M, Herring N and Patterson D. Cardiac sympatho-vagal balance and ventricular
arrhythmia (2016) Auton Neurosci 199: 29-37. https://dx.doi.org/10.1016%2Fj.autneu.2016.08.016
34. Gomes
M and Negrato C. Alpha lipoic acid as a pleotropic compound with potential
therapeutic use in diabetes and other chronic diseases (2014) Diabetol Metab
Syndr 6: 80-89. https://doi.org/10.1186/1758-5996-6-80
35. MaserR
and Lenhard M.An overview of the effect of weight loss on cardiovascular
autonomic function (2007) Curr Diabetes Rev 3: 204-211.
36. Kurpesa
M, Trzos E, Drozdz J, Bednarkiewicz Z and Krzeminska-Pakuta M.
Myocardialischmia and autonomic activity in dippers and non-dippers with
coronary artery disease: Assessment of normotensive and hypertensive patients
(2002) Int J Cardiol 83: 133-142. https://doi.org/10.1016/s0167-5273(02)00031-1
37. Eijgelsheim
M, Aamoudas A, Rivadeneira F, Kors J, Witteman J, et al. Identification of a
common variant at the NOS1AP locus strongly associated to QT-interval
prolongation (2009) Human Mol Genet 18: 347-357. https://doi.org/10.1093/hmg/ddn341
38. Rakhit
A, Maguire C, Wakimoto H, Gehrmann J, Li G, et al. In vivo electrophysiologic
studies in endothelial nitric oxide synthase (eNOS)-deficient mice (2001) J
Cardiovasc Electrophysiol 12: 1295-1301. https://doi.org/10.1046/j.1540-8167.2001.01295.x
39. Horinaka
S, Kobayashi N, Yabe A, Asakawa H, Yagi H, et.al. Nicorandil protects against
lethal ventricular arrhythmias and up-regulates endothelial nitric acid
synthase expression and sulfonylurea receptor 2 mRna in conscious rats with
acute myocardial infarction (2004) Cardiovasc Drugs Ther 18: 13-22. https://doi.org/10.1023/b:card.0000025751.82774.a9
40. Hino V, Ohkubo T, Katsubi Y and Ogawa S. Changes in endothelium-derived vascular regulatory factors during dobutamine-stress-induced silent myocardial ischemia in patients with Kawasaki disease (1999) Jpn Circ J 63: 503-508. https://doi.org/10.1253/jcj.63.503
41. Gouty
S, Regalia J, Cai F and Helke C. Alpha-lipoic acid treatment prevents the
diabetes- induced attenuation of the afferent limb of the baroreceptor reflex
in rats (2003) Auton Neurosci 108: 32-44. https://doi.org/10.1016/j.autneu.2003.08.004
42. DePace
NL and Colombo J. Autonomic and Mitochondrial Dysfunction in Clinical Diseases:
Diagnostic, Prevention, and Therapy (2019) Springer Science + Business Media,
United States.
*Corresponding author
Gary
L Murray, The Heart and Vascular Institute, 7205 Wolf River Blvd, Germantown,
TN, 38138, USA, Tel: 901-507-3100, E-mail: drglmurray@hotmail.com
Citation
Murray LG and Colombo J. Maintenance (r) alpha lipoic acid reduces
sudden cardiac death in geriatric diabetes mellitus II patients (2020) Clinical
Cardiol Cardiovascular Med 4: 6-11.
Keywords
Alpha Lipoic Acid, Diabetic Autonomic Neuropathy,
Sudden Death.


 PDF
PDF