Research Article :
Motlan and Nurdin Siregar The efficiency of
hylocereus polyrhizus based
Dye-Sensitized Solar Cell (DSSC) has been improved by the ZnO thin-film that
was used as a working electrode for DSSC. The ZnO thin-film was improved by
varying the post-heating time during the annealing process which was
synthesized by a sol-gel spin coating method. The preparation of dye solution
was conducted by cutting the hylocereus
polyrhizus into small pieces and put into a beaker glass. The hylocereus polyrhizus then was crushed
with a mortar until it was soft. In order to obtain the extracted ethanol was
added and leaf for 24 hours in a dark room. The extract then was filtered by
using filtered paper and put into a container that wrapped an aluminum foil and
kept in place to avoid the extract from sun rays. The dye sample is then UV-Vis
tested to find the highest absorbance value and wavelength of the sample. The
extract solution was used to form the ZnO/dye solar cell where the ZnO thin
film dipped into natural dyes solution with the ZnO thin films facing up for 24
hours to let the dye adsorbed by the ZnO thin film. The ZnO thin film was
dipped into extract hylocereus polyrhizus
function as a working electrode and put together with a platinum counter
electrode that separated by Surilyn. The pasting with Surilyn was conducted by
pushing the working electrode and counter electrode and heated on a hot plate
of the temperature of 70-80oC to perfectly put together. The working
electrode which was put together to the platinum counter electrode was injected
with liquid electrolytes through a small hole on the platinum counter
electrode. Electrical testing is carried out after the DSSC has been assembled
by making an electrical circuit between the DSSC with a measuring instrument.
The sensitizer value of the hylocereus
polyrhizus was 0.652 au, at the wavelength of 538 nm. The maximum power of
the DSSC was 0.10030 w/cm2 and the efficiency of 0.0274%. Sunlight is one of the energy
sources that have an abundant energy source; however, it has not been exploited
well. The energy released by angry rays is actually only received by the
surface of the earth at 69% of the total solar radiant energy [1]. The supply
of solar energy from sunlight received by the earth s surface reaches 3 x 1024
J/year, this energy is equivalent to 2 x 1017 watts. The amount of energy is
equivalent to Ten Thousand times the energy consumption throughout the world.
In other words, by closing only 0.1% of the earth s surface with solar cell
devices that have 10% efficiency is able to meet the energy needs of the world.
Solar cells are devices that convert solar energy into electrical energy, which
is directly the current and voltage produced by solar cells depending on
sunlight. Solar cells based on current
technological developments and manufacturing materials can be divided into
three, namely: First, solar cells made of single silicon and multi-crystalline silicon.
Second, thin-film solar cells and the third organic solar cells DSSC, the third
generation of chip and reliable conversion of solar energy has gained an
attraction to the development of thin-layer solar cells [2,3]. DSSC consists of
several components such as oxide semiconductor, dyes, the counter electrode and
electrolyte. There are several ways of improving the quality of the DSSC such
as obtaining a growth window of the thin film semiconductor, the molecular structure
of the dyes, electrolyte pair of reduction-oxidation and
electrode material. In the engineering of the semiconductor that is used as
working electrode in the DSSC, the use of ZnO thin film has given high promise
due to its higher bandgap compared to TiO2 that previously used,
therefore gives higher voltage, higher carrier mobility that can reduce carrier
recombination, and has structure and morphology that can be
controlled in its synthesis. In addition, ZnO also has a wide bandgap of 3.37
eV, has high optical transparency at room temperature and 60 meV electron
binding ability [4,5]. ZnO thin film has been synthesized by several methods such
as molecular beam epitaxy, RF magnetron sputtering, pulsed laser deposition,
spray pyrolysis, chemical bath deposition, physical vapor deposition [6-14]. However, this method involves a
rather complicated process carried out because it requires sophisticated equipment,
instead of using the sol-gel spin coating method because the equipment is
simple and low cost, does not use space with high vacuum and very good
microstructure. Research on DSSC has been carried out using various types of semiconductors with
variations in heating temperature, heating holding time, spin-coating speed and
various types of dyes using thin-film TiO2 with calcination temperature of 450oC and holding time 120 minutes and using dye
extract from spinach leaves, DSSC efficiency was obtained 0.13% [15]. Thin-film TiO2 with
calcination temperature of 450oC and dye extract from Male Flower
Leaves (Luffa Cylindra-L) obtained efficiency DSSC 1.3% [16]. Thin-film TiO2
and dye extracts from various fruits and leaves, the result is that the
magnitude of DSSC efficiency depends on the fruit or leaf extract used [17,18].
TiO2 thin films and the variation of solvents in dye extracts from Melastoma Malabathricum
Leaves fruit results that the magnitude of DSSC efficiency was influenced by
solvents [19]. Varying the thickness of TiO2 thin films the result
is that the magnitude of DSSC efficiency is influenced by the thin film thickness
[20]. Variations in the temperature of ZnO thin films (300 oC, 350
oC, 400 oC and 500oC), the result is the highest
DSSC efficiency of 3.92% at 400oC [21]. It cause ZnO has demonstrated
multifunctional properties with high energy binding strength, low resistivity,
great light catching characteristics, high optical transparency at room
temperature, 3.37 eV wide bandgap, and the ability to bind free electrons at 60
meV. The quality of the thin film is a crucial point in the improvement of the
efficiency of the DSSC. In this work, we tried to improve the quality of the
ZnO thin film by varying the Post-Heating time of the ZnO thin film during the
sol-gel spin coating. The aim of this research is seeing the effect of
Post-Heating time of ZnO thin film on ZnO/dye-based DSSC efficiency. ZnO Thin Film Synthesis Materials used in this research were
Zinc Acetate Dehydrate, Isopropanol and Diethanolamine which
successively used as basic materials, solutions, and stabilizers. The ZnO thin
film was synthesized by using sol-gel spin coating method. Zinc Acetate
dehydrates (Zn(CH3COOH).2H2O) was diluted in isopropanol, stirred
with a magnetic stirrer for 10 minutes, then little by little 1.72 ml
Diethanolamine (DEA) was dropped into the solution. The solution which was in
the form of the gel was dropped on an FTO glass substrate and spin with a speed
of 5000 rpm. The sample then was heated with pre-heating 250oC and Post-Heating 500oC with heating with a
variation of 30, 60, 90, 120 and 150 minutes. The ZnO thin film samples were
then characterized by (X-Ray Diffraction) XRD, SEM, and UV-Vis. The preparation of dye solution was
conducted by cutting the hylocereus polyrhizus into
small pieces and put into a beaker glass. The hylocereus polyrhizus then was crushed with a mortar until it was
soft. In order to obtain the extracted ethanol was added and leaf for 24 hours
in a dark room. The extract then was filtered by using filtered paper and put
into a container that wrapped an aluminum foil and kept in place to avoid the
extract from sun rays. The dye sample is then UV-Vis tested to find the highest
absorbance value and wavelength of the sample. The extract solution was used to form the ZnO/dye solar cell
where the ZnO thin film dipped into natural dyes solution with the ZnO thin
films facing up for 24 hours to let the dye adsorbed by the ZnO thin film. The
ZnO thin film was dipped into extract hylocereus
polyrhizus function as a working electrode and put together with a platinum
counter electrode that separated by Surilyn. The pasting
with Surilyn was conducted by pushing the working electrode and counter
electrode and heated on a hot plate of the temperature of 70-80oC to perfectly put together. The working electrode which was
put together to the platinum counter electrode was injected with liquid
electrolytes through a small hole on the platinum counter electrode. Electrical testing is carried out after the DSSC has been
assembled by making an electrical circuit between the DSSC with a measuring
instrument, namely a digital multimeter as shown in Figure 1. This test is based on the lighting method of the light
beam to determine the performance and efficiency of the cells obtained when the
solar cell object is exposed to light with certain intensity at the top of the
electrode (anode). DSSC outputs are an Open-Circuit Voltage
(Voc) and Short Circuit Current (Isc) DSSC. Then the amount of fill factor (FF)
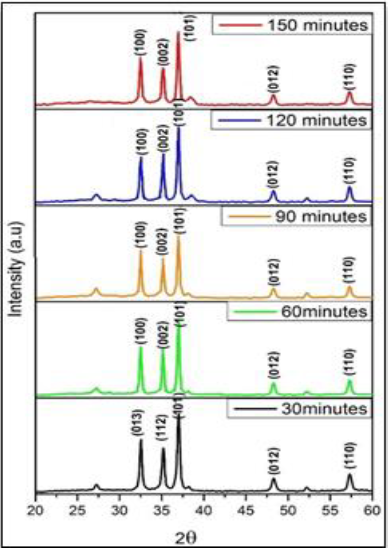
and DSSC efficiency are calculated (η). Figure 1: DSSC Efficiency Measurement. The crystal structure was determined by using XRD. Figure 2 shows the XRD pattern of the
ZnO thin film of a sample with pre-heating 250oC and Post-Heating
500oC with the variation of 30, 60, 90, 120 and 150 minutes. Figure 2: X-ray diffraction spectra of ZnO. The X-ray diffraction pattern of the sample is analyzed
using search march by showing the sample has the same crystal planes namely
fields (100), (002), (101) and has the same growth peak and oriented to the
plane (101). Analysis of the XRD pattern shows that all samples are hexagonal
wurtzite and growth orientation toward the C axis perpendicular to the substrate
surface along with standard data of ZnO of JCPDS 80-0075 card. The ZnO crystal
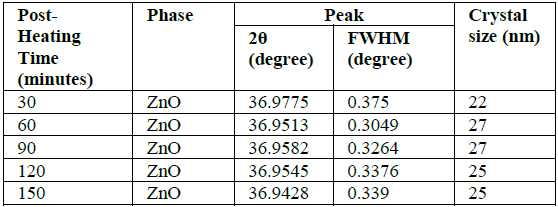
size measured using Scherrer equation 1 [22]. D=
0.9 λ/ β cos θ (1) Whereas D=crystal size, λ=wavelength, β=FWHM (Full Width
Half Maximum), θ=diffraction angle. Crystal sizes of the ZnO thin films calculated using equation
1 are shown in Table 1. The size of the crystal is influenced by the value of FWHM,
if the FWHM value decreases the size of the crystal is large and vice versa.
The size of the crystals obtained for all samples is almost the same in the
range of 22-27 nm. This shows that the duration of heating does not affect the
size of the crystal, because the difference in the duration of heating is very
small. The increase in Post-Heating
temperature is in line with the decreasing FWHM value and increasing
crystal size, this is due to the higher heating temperature in the growth of
ZnO thin film crystals, the energy obtained by ZnO atoms to form the higher the
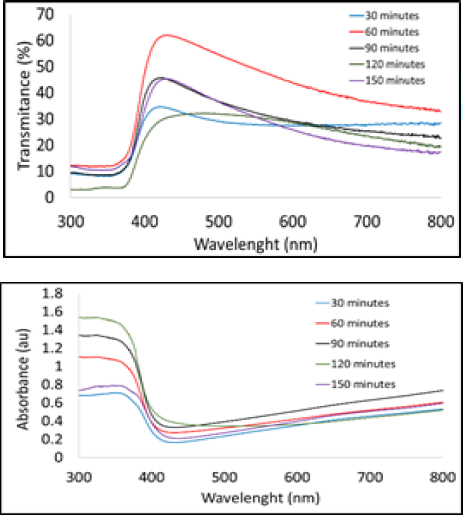
crystal field as well, so the better the Crystal formed [19-23]. Transmittance and absorption spectra for all samples were
taken in the wavelength range of 300-800 nm which is in the range of its
application for solar cells. Figure 3a
shows there is a sharp increase in transmission of the ZO thin film in the
wavelength range of 350-430 nm which is in the ultraviolet range. The highest
and lowest transmission value is when the Post-Heating of 30 and 120 minutes.
According to the heating mechanism the higher, the heating temperature and the
longer the Post-Heating time the better is the compacting of the powder and the
stronger the bond among granules
and porosity of the material. The high transmission value of ZnO thin film, make it a good
application for solar cell [24]. Absorbance spectrum on the ZO thin film is
shown in Figure 3b. There is a sharp
decrease in the adsorption for all samples in the wavelength range of 350-430
nm and the highest and lowest adsorption consecutively when Post-Heating 120
and 30 minutes. Therefore, the ZnO thin film has high transmittance at visible
light and can be applied in optoelectronic. The ZnO thin film has a direct
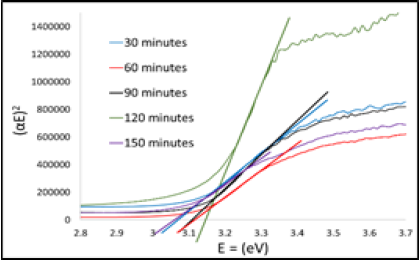
bandgap, therefore the bandgap can be calculated by using the following equation
2 [25,26]. The bandgap with variations in the
duration of heating to Post-Heating of various Post-Heating time of ZnO thin
film which was derived using the Tauc Plot method is shown in Figure 4. Figure 4: The bandgap of ZnO using Tauc Plot method. The bandgap of Figure 4 of ZnO thin
film as a function of Post-Heating. Figure 4 shows that the highest bandgap is
3.140 eV and the lowest is 3,073 eV of Post-Heating time of each 120 and 150
minutes. Generally, the bandgap increases as the Post-Heating time increase up
to 120 minutes, beyond that temperature the band gap decreases. The increase in
bandgap along with the increase in Post-Heating time is due to the compacting
of the granules, and the decrease of porosity size and number [27-29]. The
increase in density of the smaller granules will increase the surface energy as
well as the bandgap. However, by further increasing the Post-Heating time will
start forming bigger granules and reduce the energy gap [30]. The absorption of the sensitizer
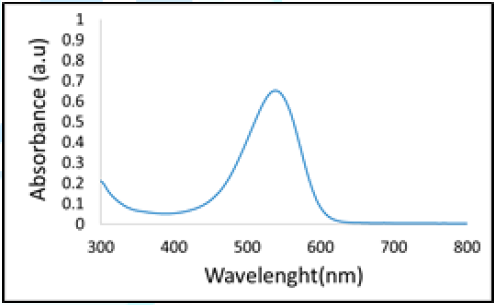
extracted red dragon dye solution which was determined by UV-Vis is shown in Figure 5. Figure 5: Graph of wavelength Vs absorption of extracted hylocereus polyrhizus dye solution. The absorption intensity of the hylocereus polyrhizus dye solution as
shown in Figure 5 is 0,652 a.u at the wavelength of 538 nm. Previous work
showed that the peak absorption of the hylocereus
polyrhizus was in the range of 420-580 and absorption intensity was
affected by solution concentration, the higher the solution concentration the
higher is the solution absorption value [31,32]. Absorption peaks in the range
of the wavelength of 500-600 nm show that there is the content of anthocyanin in the dye
solution [33-35]. The peak absorption of hylocereus
polyrhizus at a wavelength of 535 nm which is in the range of 450-600 nm
[36]. The photon absorption by the solar cell is very crucial in solar cell
technology, the higher the absorption the bigger the photon excitation the
bigger is the amount of solar energy converted into electricity. The percentage of efficiency is
obtained by comparing the power produced by the DSSC (Pmax) prototype with the
power which is given by solar origin (Pin) shown by the following equation 3 and
the output power can be obtained from the following equation 4. ƞ (%) = ((J_SC) x
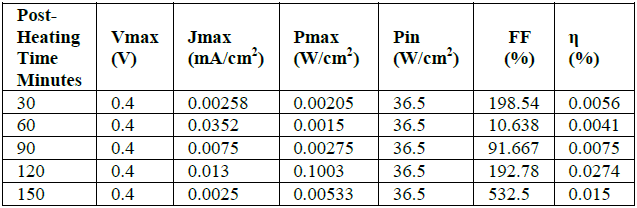
(V_OC) x FF x 100)/P_in (3) P_max=V_max x J_max (4) Table 2 Shows the efficiency ZnO/DSSC of
the hylocereus polyrhizus based on a
variation of Post-Heating time during the synthesis of ZnO thin film is shown
in Table 2. Table 2 shows that the highest efficiency of the DSSC is
when the Post-Heating time of the ZnO is 120 minutes which 0.0274%. The increase
in the bandgap, as well as the increase in energy efficiency, is due to the
improvement in crystal growth during the Post-Heating time. The increase in the
formation of small granules, and at the same time decrease the porosity has
improved the quality of the DSSC. The increase in the number of granules will
facilitate electrons flow from conduction band into valence band that
facilitates photocatalyst
reaction and bigger absorption by the dye, and therefore bigger spectrum. Semiconductors
with a wide bandgap will multiply electrons flowing from the conduction band to
the valence band, which makes the photocatalyst reaction chamber and absorption
by the dye will become more so that the spectrum becomes wide [37]. Dye-Sensitized Solar Cell can be fabricated by using ZnO
thin film with the variation of Post-Heating and extracted dye from hylocereus polyrhizus . The smallest
size of the ZnO thin-film was 22 nm for Post-Heating of 30 minutes and the
bandgap 3.140 eV for the Post-Heating time of 120 minutes. The intensity of
extracted dye from the hylocereus polyrhizus
Sensitizer is 0.652 a.u and peak position at 538 nm. The ZnO thin-film and hylocereus polyrhizus based DSSC can
convert solar energy into electrical energy. The maximum power obtained in this
research was 0.10030 W/cm2 and the maximum efficiency of 0.0274% for
the Post-Heating time during synthesis ZnO thin-film of 120 minutes. 1. Girard EJ. Principles of Environmental
Chemistry (2nd Edn) (2010) Massachusetts: Jones and Bartlett
Publishers, Massachusetts, USA. 2. Grätzel C and Zakeeruddin SM. Recent
trends in mesoscopic solar cells based on molecular and nanopigment light
harvesters (2013) Materials Today 16: 11-18. https://doi.org/10.1016/j.mattod.2013.01.020 3. Wang M, Grätzel C, Zakeeruddin SM
and Grätzel M. Recent developments in redox electrolytes for dye-sensitized
solar cells (2012) Energy and Environmental Sci https://doi.org/10.1039/c2ee23081j 4. Ernawita E, Irwansyah I, Sawitri D
and Wahyuono RA, Preparasi dan Karakterisasi Dye-sensitized Solar Cell (DSSC)
dengan Pewarna Ekstrak Jeruk: Pengaruh Variasi Komposisi Karotenoid dan
Flavonoid Terhadap Efisiensi Sel Surya (2017) J. Fis. dan Apl. 13: 3. https://doi.org/10.12962/j24604682.v13i3.2839 5. Siregar N and Motlan. The Effect of
Pre-heating Temperature on Structural and Optical Properties ZnO Thin Film
Synthesized using Sol-Gel spin Coating Method (2018) Journal of Physics:
Conference Series 1120. https://doi.org/10.1088/1742-6596/1120/1/012088 6. Wang C, Chen Z, Hu H and Zhang D. Effect
of the oxygen pressure on the microstructure and optical properties of ZnO
films prepared by laser molecular beam epitaxy (2009) Phys. B Condens. Matter
404: 4075-4082. https://doi.org/10.1016/j.physb.2009.07.165 7. Kumar Y, Escorcia GJ, Singh F, Olive-Méndez
SF, et al. Influence of mesoporous substrate morphology on the structural,
optical and electrical properties of RF sputtered ZnO layer deposited over
porous silicon nanostructure (2012) Appl. Surf. Sci 258: 2283-2288. https://doi.org/10.1016/j.apsusc.2011.09.131 8. Zhu BL, Zhao X, Su F, Li GH, et al. Low
temperature annealing effects on the structure and optical properties of ZnO
films grown by pulsed laser deposition (2010) Vacuum 84:1280-1286. 9. Terasako T, Uno Y, Inoue S, Kariya T
and Shirakata S. Structural, optical and electrical properties of CuInS2 thin
films prepared by chemical spray pyrolysis (2006) Physica Status Solidi (C)
Current Topics in Solid State Physics 3: 2588-2591. https://doi.org/10.1002/pssc.200669597 10. Falcade T, Oliveira DBG, Bergmann
CP, Müller IL and Fraga DMC. The effects of spray pyrolisis parameters on the
morphology and microstructure of the YSZ film deposited on LSM cathode (2010)
65th ABM International Congress, 18th IFHTSE Congress and 1st TMS/ABM
International Materials Congress 727: 691-696. https://doi.org/10.4028/www.scientific.net/msf.727-728.691 11. Cubillos GI, Olaya JJ, Bethencourt
M, Cifredo G and Blanco G. Structural changes in ZrOxNy/ZrO2 coatings deposited
through spray pyrolisis-nitriding (2014) Rev Mex Fis 60: 233-242. 12. Kumar PS, Raj AD, Mangalaraj D and
Nataraj D. Growth and characterization of ZnO nanostructured thin films by a
two-step chemical method (2008) Appl Surf Sci 255: 2382-2387. https://doi.org/10.1016/j.apsusc.2008.07.136 13. Mane RS, Lee WJ, Pathan HM and Han
SH. Nanocrystalline TiO2/ZnO thin films: Fabrication and application
to dye-sensitized solar cells (2005) J Phys Chem B 109: 24254-24259. https://doi.org/10.1021/jp0531560 14. George A, Kumari P, Soin N, Roy SS
and McLaughlin JA. Microstructure and field emission characteristics of ZnO
nanoneedles grown by physical vapor deposition (2010) Mater Chem Phys 123:
634-638. https://doi.org/10.1016/j.matchemphys.2010.05.029 15. Chang H, Wu HM, Chen TL, Huang KD,
Jwo CS, et al. Dye-sensitized solar cell using natural dyes extracted from
spinach and ipomoea (2010) J Alloys Compd 495: 606-610. https://doi.org/10.1016/j.jallcom.2009.10.057 16. Maurya IC, Srivastava P and Bahadur
L. Dye-sensitized solar cell using extract from petals of male flowers Luffa
cylindrica L. as a natural sensitizer (2016) Opt Mater (Amst) 52: 150-156. https://doi.org/10.1016/j.optmat.2015.12.016 17. R Ramamoorthy, N Radha, G Maheswari,
S Anandan, S Manoharan, et al. Betalain and anthocyanin dye-sensitized solar
cells (2016) J Appl Electrochem 46: 929-941. https://doi.org/10.1007/s10800-016-0974-9 18. Lim A, Kumara NTRN, Ling TA, HuqMirza
A, Chandrakanthi RLN, et al. Potential natural sensitizers extracted from the
skin of Canarium odontophyllum fruits for dye-sensitized solar cells (2015)
Spectrochim. Acta-Part A Mol Biomol Spectrosc 138: 596-602. https://doi.org/10.1016/j.saa.2014.11.102 19. Singh LK, Karlo T and Pandey A. Performance
of fruit extract of Melastoma
Malabathricum L. as sensitizer in DSSCs (2014) Spectrochim. Acta-Part A Mol
Biomol Spectrosc 118: 938-943. https://doi.org/10.1016/j.saa.2013.09.075 20. Kao MC, Chen HZ, Young SL, Kung CY and
Lin CC. The effects of the thickness of TiO2 films on the
performance of dye-sensitized solar cells (2009) Thin Solid Films 517:
5096-5099. https://doi.org/10.1016/j.tsf.2009.03.102 21. Lu L, Li R, Fan K and Peng T. Effects
of annealing conditions on the photoelectrochemical properties of
dye-sensitized solar cells made with ZnO nanoparticles (2010) Sol. Energy 84:
844-853. https://doi.org/10.1016/j.solener.2010.02.010 22. Cullity DB and Stock RS. Elements of
x-ray diffraction (3rd Edn) (2014) Pearson Education India, India. 23. HA Dehkordi, K Dastafkan, A Moshaii
and A Mokhtari. Thermal post-annealing and gas concentration effect on liquid
petroleum gas sensing characteristics of nanocrystalline zinc oxide thin films
(2015) J Mater Sci Mater Electron 26: 3134-3142. https://doi.org/10.1007/s10854-015-2808-7 24. Sathya M, Claude A, Govindasamy P, Sudha
K and Claude A. Growth of pure and doped ZnO thin films for solar cell
applications (2012) Pelagia Res Libr Adv Appl Sci Res 3: 2591-2598. 25. Lee JC, Kang KH, Kim SK, Yoon KH,
Park IJ, et al. RF sputter deposition of the high-quality intrinsic and n-type
ZnO window layers for Cu(In,Ga)Se2-based solar cell applications (2000) Sol
Energy Mater Sol Cells. 64: 185-195. https://doi.org/10.1016/s0927-0248(00)00069-6 26. Sridev D and Rajendran KV. Synthesis
and optical characteristics of ZnO nanocrystals (2009) Bull Mater Sci 32:
165-168. 27. Das MD, Dhakal A and Shah BR. Effect of Annealing on Optical Properties
of Zinc Oxide Thin Films Prepared by Homemade Spin Coater (2015) Nepal J Sci
Technol 15: 111-116. https://doi.org/10.3126/njst.v15i2.12126 28. Yadav AB, Pandey A and Jit S. Effects
of annealing temperature on the structural, optical, and electrical properties
of ZnO thin films grown on n-Si〈100〉 substrates by the sol-gel spin
coating method (2014) Acta Metall Sin (English Letters) 27:682-688. https://doi.org/10.1007/s40195-014-0097-4 29. Kumar S, Basu S, Rana B, Barman A, Chatterjee
S, et al. Structural, optical and magnetic properties of sol-gel derived ZnO:Co
diluted magnetic semiconductor nanocrystals: An EXAFS study (2014) J Mater Chem
C 2:481-495. https://doi.org/10.1039/c3tc31834f 30. Motlan and Goldys EM. Photoluminescence
of multilayer GaSb/GaAs self-assembled quantum dots grown by metalorganic
chemical vapor deposition at atmospheric pressure (2001) Appl Phys Lett 79:
2976. https://doi.org/10.1063/1.1415351 31. Naderi N, Ghazali HM, Hussin ASM,
Amid M and Mohd MY. Characterization and quantification of dragon fruit (Hylocereus polyrhizus ) betacyanin pigments
extracted by two procedures (2012) Pertanika J Trop Agric Sci 35: 33-40. 32. Gomesh N, Syafinar R, Irwanto M, Irwan
YM, Fareq M, et al. Characteristics of Dye-Sensitized Solar Cells Using Dye
from Pitaya Fruit (2015) Appl Mech Mater 793: 450-454. https://doi.org/10.4028/www.scientific.net/amm.793.450 33. Lai WH, Su YH, Teoh LG and Hon MH. Commercial
and natural dyes as photosensitizers for a water-based dye-sensitized solar cell
loaded with gold nanoparticles (2008) J Photochem Photobiol A Chem 195:
307-313. https://doi.org/10.1016/j.jphotochem.2007.10.018 34. Hemalatha KV, Karthick SN, Justin
Raj C, Hong NY, Kim SK, et al. Performance of Kerria japonica and Rosa
chinensis flower dyes as sensitizers for dye-sensitized solar cells (2012)
Spectrochim Acta-Part A Mol Biomol Spectrosc 96: 305-309. https://doi.org/10.1016/j.saa.2012.05.027 35. Yusoff A, Kumara NTRN, Lim A,
Ekanayake P and Tennakoon KU. Impacts of temperature on the stability of
tropical plant pigments as sensitizers for dye sensitized solar cells (2014) J
Biophys 2014: 739514. https://doi.org/10.1155/2014/739514 36. Ali R and Nayan N. Fabrication and
analysis of dye-sensitized solar cell using natural dye extracted from dragon
fruit (2010) Int J Integrated Eng 2: 55-62. 37. Motlan and Siregar N. The Effect of
Post-Heating Temperature on of the Eficency of Dye Sensitized Solar Cell (DSSC)
with Using ZnO Thin Film and Dye from Dutch Eggplant Fruit (Solanum betaceum)
(2018) J Physics: Conference Series 1120. https://doi.org/10.1088/1742-6596/1120/1/012082 Motlan, Faculty
of Mathematics and Natural Sciences, Universitas Negeri Medan, Medan,
Indonesia, Email: motlan@unimed.ac.id Motlan and Siregar N. The effect of post-heating
time of ZnO thin film on the efficiency of ZnO/hylocereus polyrhizus dssc (2019) Edelweiss Appli Sci Tech 3:
70-74. Dye-sensitized solar cell, Post-heating time,
ZnO thin-film, Sol-gel spin coating method, Hylocereus polyrhizus.The Effect of Post-Heating Time of ZnO Thin Film on the Efficiency of ZnO Hylocereus polyrhizus DSSC
Abstract
Full-Text
Introduction
Experiment Method
The Preparation of Dye Solution (Sensitizer)
DSSC Synthesis
Efficiency Measurement

Result and Discussion
The Optical Property of the ZnO Thin
Film
The Absorbance of Hylocereus
polyrhizus Dye Solution
The efficiency of DSSC
Conclusion
References
Corresponding author:
Citation:
Keywords