Research Article :
A
novel simple and selective electrochemical procedure is utilized for the
determination of Dinoprostone (DIN) in drug substance and pharmaceutical
preparation with good recovery and without interference with other excipient.
Herein, the electrochemical sensing platform based upon preparing gold
nanoparticle sensor on silica modified carbon paste electrode. The surface
morphology of the modified electrode was characterized by scanning electron
microscope. Different experimental conditions, including electrode composition,
effect of pH and scan rate were estimated carefully by cyclic voltammetry to
obtain the highest electrochemical response. By using square wave voltammetry a
good linear response was obtained in the range of, 2 x 10-5-4 x10-4
mol L-1, and 2 x 10-7-1.6 x 10-4 mol L-1,
with low detection limit of 5 x 10-6 mol L-1, and 4.9 x
10-8 mol L-1 by CPE and GNP/SMCPE respectively. The
obtained results are in good agreement with those obtained by official method.
No electrochemical method was reported before for determination of DIN. The
developed method was simple, rapid, economic and challenging to green
analytical chemistry. Prostaglandins are
essential mediator that are formed in many tissues and adjust many
physiological functions, over normal and/ or patho physiological conditions [1-3].
They have many functions, as, the role of bone cells in establishing the
hematopoietic stem cell, immunotherapy of cancer, female reproduction, platelet
receptors, type I collagen structure, synthesis, and regulation nonsteroidal
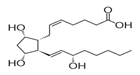
anti-inflammatory drugs for osteoarthritis [4] (Figure 1). Figure 1: Dinoprostone
(DIN) chemical structure. Dinoprostone (DIN) in
medicine identified as Prostaglandin E2, (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxyoct-1-enylcyclopentyl]
hept-5-enoic acid is a naturally occurring prostaglandin used in medicine to
induce labor and as an abortifacient [5,6].
Dinoprostone stimulates myometrial contractions in the gravid uterus that are
similar to the contractions that occur in the term uterus during labor [7,8]. These
contractions are usually sufficient to cause abortion [9]. Few
analytical methods were developed and validated for determination of
dinoprostone in drug substance, dosage form, human gastric mucosa, and in
cultured tumor cells using HPLC with UV, laser induced fluorescence and
electrospray ionization tandem mass spectrometric detectors. GC-MS was used
also for determination of the drug in cultured tumor cells [10]. Due
to the important biological role of prostaglandins, fast, simple and sensitive electrochemical
method has to be developed for determination of DIN. Carbon Paste Electrodes
(CPE) has been widely applied in the field of electrochemistry for the
determination of low analyte concentrations due to their ease of fabrication,
low cost and high sensitivity [11]. Modification
of electrodes with various modifiers has been reported in recent years to
improve sensitivity, selectivity, and detection limit [12-14]. Silica gel can
be incorporated in paste with carbon and used as modifier. It has high
adsorption capacity, insolubility in most solvents, and thermal stability, and
high surface area of synthetic silica makes it valuable as support for various
catalysts [15-20]. Gold
Nanoparticles (GNPs) with large surface area, good biocompatibility, and
high conductivity and electro catalytic activity have been used to increase
sensitivity and improve detection limits [21-27]. The
literature survey revealed that no attempt had been made to study the
voltammetric behavior of dinoprostone. Therefore, the aim of the present work
was to prepare a new sensor based on gold nanoparticles, silica, and graphite
for rapid and selective electro analytical determination of DIN in drug
substance and pharmaceutical
product. Moreover the prepared electrode was characterized and the surface
area was calculated. Materials
and reagents Dinoprostone
was kindly supplied from Amriya Pharmaceutical Co., Egypt, and its purity was
found to be 98.53% according to USP Pharmacopiea. Dinoglandin E2 (batch NO.
09477, Alexandria Co. for Pharmaceutical and Chemical Industeries) was labeled
to contain 3 mg DIN per vaginal tablet. It was purchased from the local market.
Silica gel was purchased from Sd. fine Chem. Ltd. Mumbai. Hydrogen
tetrachloroaurate (HAuCl4) across organics New Jersey batch NO.
AO321694 was purchased from Sigma-Aldrich. Britton-Robinson buffer (B-R buffer)
was prepared by mixing different volumes of 0.04 M in H3PO4
(Adwic Co., Egypt), 0.04 M acetic acid (LOBA-Chemic Co., India), and 0.04 M
boric acid (Polski EODZNN Chemiczne S.A. Co., Poland) with the appropriate
amount of 0.2 M NaOH (Adwia Co., Egypt) to obtain the desired pH of 2.0-9.0.
Buffer solutions were kept in a refrigerator [28]. All solutions were prepared
from chemicals of analytical grade, and sterilized Milli-Q deionized water was
used. Standard
solutions Stock
standard solution of dinoprostone (1 × 10-2 M) was prepared by
dissolving appropriate amount of the drug in deionized water. Preparation
of electrodes Carbon
Paste Electrode (CPE) was prepared by mixing graphite powder (0.5 g) with
paraffin oil (0.3 mL) in a glassy mortar. The carbon paste was packed into the
hole of the electrode body and smoothed on a filter paper until its shiny
appearance. Modified silica gel CPE (SMCPE) was prepared by mixing graphite
powder with 5 % of its weight with silica gel. For better homogeneity, the
resulting composite was dispersed in ethanol and stirred on a magnetic stirrer
until the solvent completely evaporated, then about 3 mL of paraffin oil was
added. Gold
silica modified electrode was prepared by immersing silica gel-modified CPE
(SMCPE) composite into 6 mM hydrogen tetrachloroaurate (HAuCl4)
solution containing 0.1 M KNO3 [29]. All the prepared electrodes
were washed with double distilled water and dried carefully with a paper
without touching the surface and then left to dry in air for 10 min before
being used. Instrumental
and experimental setup All
voltammetric measurements were performed using A Bio-logic SP 150 electrochemical
workstation. A One compartment cell and the three electrodes were connected to
the electrochemical workstation through a C3-stand. A platinum wire from BAS
(USA) was employed as the auxiliary electrode. The electrode potentials were
measured with respect to the reference electrode Ag/AgCl electrode from BAS
(USA). Sigma Plot 11 was used for the transformation of the initial signal. A
Cyberscan 500 digital (EUTECH Instruments, USA) pH meter with a glass
combination electrode served to carry out the pH measurement. Scanning Electron
Microscopy ( Electroanalytical
measurements Construction
of calibration curve of dinoprostone; Aliquots equivalent to (0.1-2.0 mL), and
(1-800 μL) from 1 × 10-3 M solutions of DIN were transferred into a
series of 5-mL volumetric flasks for CPE and GNP/SMCPE respectively, using
micropipette, and the volume was completed to the mark with B-R buffer pH 2.
This solution was transferred to the electrolytic cell, and then Square Wave
Voltammogram (SWV) was recorded. The peak current was measured at a scan rate
of 10 mV s-1 using gold nanoparticles silica gel-modified CPE
(GNP/SMCPE). Calibration curve was constructed by plotting the peak currents
against drug concentrations. Application
to pharmaceutical product Commercial
pharmaceutical samples containing DIN was analyzed to evaluate the validity of
the proposed method. Five vaginal tablets were finely mixed, and a weight
equivalent to 15 mg of dinoprostone
was dissolved in 30 mL of deionized water. Then 1.4 mL was transferred
quantitatively to a 100 mL volumetric flask and completed to the mark with
deionized water to obtain 10-3 M. Appropriate dilutions with
deionized water were done to prepare samples in the quantification range. Morphologies
of different electrodes The
response of an electrochemical
sensor was related to its physical morphology. The morphology of bare CPE (A),
SMCPE (B), and GNP/SMCPE (C) were shown in Figure
2. Figure 2: Scanning
electron microscope images of (A) bare CPE, (B) SMCPE and (C) GNP/SMCPE. The
SEM image of CPE shows that its surface was characterized by a compact surface,
isolated and irregularly shaped graphite, while the SEM image of GSMCPE shows
that metallic nanoparticles are located at different elevations over the
substrate. Moreover, a porous nanostructured film of gold nanoparticles was
noticed which extremely enhanced the active surface area of GNP/SMCPE and might
be very important to promote electron transfer. Electrochemistry
of dinoprostone Preliminary
investigation using cyclic voltammetry shows a behavior of irreversible
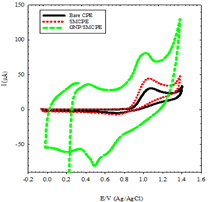
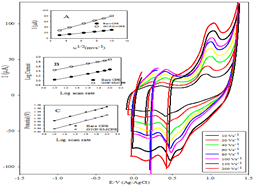
oxidation of DIN at bare CPE, SMCPE, and GNP/SMCPE. Figure 3 shows typical cyclic voltammograms of 1.0 × 10-2
mol L-1 of DIN, in B-R buffer pH 2.0, at a scan rate of 100 mV s-1,
recorded at three electrodes under investigation. At bare CPE, the oxidation
peak current was observed to be 30.4 μA, while in the case of SMCPE, the
oxidation peak current was found to be 40.1 μA and the best one is GNP/SMCPE,
which has a value of 80 μA. The
potential of different electrodes were found in order of, bare CPE, SMCPE, and
GNP/SMCPE respectively (1.13 V compared to 1.12 V, and 1.16 V), due to the
improvement in the reversibility of the electron transfer process and a larger
real surface area of the modified electrode. The electro deposition of gold
nanoparticles on GNP /SMCPE resulted in an observable increase in the peak
current, which indicated an improvement in the electrode kinetics and increase
in the potential of oxidation substantial, where GNP/SMCPE acts as a cation
exchange [29,30] that attracts the positively charged DIN. Selection
of the Optimum Experimental Conditions Effect of pH: In order to
ascertain that electrocatalytic oxidation of DIN would be pH dependent, the
voltammetric response of DIN was investigated in solutions with varying pH from
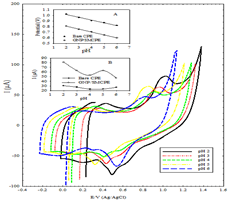
2.0 to 6.0 in order to optimize the electrocatalytic response. Figure 4 shows the cyclic voltammograms
of the oxidation peak currents of DIN at different pH values using B-R buffer
at bare CPE, and GNP/SMCPE electrode. Higher anodic current for DIN at pH 2 is
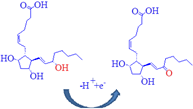
due to major microspecies at this pH. Moreover,
DIN oxidation is a one-electron process, which may be attributed to the
oxidation of double bonds [31-33]. DIN carries positive charge that can be
attracted by the negative charge of the electrode; the suggested
electrochemical oxidation of DIN was depicted in Scheme 1. Scheme 1: Suggested
oxidation mechanism of dinoprostone. A
comparison between the anodic peak current at different pH values of bare CPE,
and GNP/SMCPE show that GNP/SMCPE displays higher anodic current for DIN than
bare CPE which indicates the effect of gold on the catalytic oxidation
processes as shown in Figure 2. It is observed that as the pH values increase,
the peak potential shifts toward less positive values, which indicates the
participation of protons in the electrode process and that the electrocatalytic oxidation
of DIN is a pH-dependent reaction. The relationship between the anodic peak
potential and the solution pH value at bare CPE and GNP/SMCPE could be fit to
the linear regression equation of Epa (V)=1.1132-0.0493 pH, with a
correlation coefficient of r=0.9973 and Epa (V)=0.9118-0.0521 pH,
with a correlation coefficient of r = 0.9994 respectively. The slope was found
to be 49.3 mV/pH and 52.1 mV/pH units at bare CPE and GNP/SMCPE respectively
over the pH range from 2 to 6, which is close to the theoretical value of -48.3
mV. This indicated that the number of protons and transferred electrons
involved in the oxidation mechanism are equal [34]. Effect of scan
rate: The
interfacial reaction of the drug at each electrode was identified by recording the
cyclic voltammograms of 1 × 10-3 M solution at different scan rates
(ν) 10-250 mV s-1 in B-R buffer (pH 2.0). Typical CV curves of DIN
at different scan rates were shown in Figure
5. Figure 5: Cyclic
voltammograms of 1.0 × 10-3 mol L-1 DIN at GNP/SMCPE in
0.04 M B-R buffer pH 2 from 10 to 200 mV s-1, Inset A: plot of Ip
vs. v1/2. Inset B: plot of log Ip vs. log v. Inset C:
plot of Ep vs. log v. Figure
5 inset A showed that the peak current increased linearly with increasing the
square root of scan rate up to a scan rate of 100 mV s-1, according
to regression equation [35]: ip= (2.69 x 105) n 3/2 A Co
x Do ½ ν1/2 In
this equation, ip is the peak current density (μA cm-2),
n is the number of electrons appearing in half-reaction for the redox couple, ν
is the scan rate at which the potential is swept (V s-1), C is the
analyte concentration, A is the electrode area (0.071 and 0.118 cm2
for bare CPE, and GNP/SMCPE respectively), and Do is the
electroactive species diffusion coefficient (cm2 s-1).
The apparent diffusion coefficient, Dapp, of DIN in B-R buffer (pH 2) was
calculated from Cyclic
Voltammetry (CV) experiments which increases from 8.9 × 10-7 cm2
s-1 in case of using bare CPE to 4.3 × 10-5 cm2 s-1
after the functionalization of bare CPE surface with gold nanoparticles. This
indicated the quick mass transfer of the analyte molecules toward GNP/SMCPE
surface from bulk solutions and fast electron transfer process of
electrochemical oxidation of the analyte molecule at the electrode-solution
interface. The calculated Dapp values also showed that gold improves
the electron transfer kinetics at the electrode/solution interface, suggesting
that the reaction is a diffusion-controlled electrode reaction. Direct
proportionality was obtained between log current and log scan rate in range of
10-100 mV s-1 (Figure 5 inset B), giving the following equation: log I=0.6003+0.435 log ν r=0.9994 for Bare CPE log I=1.0134+0.444 log ν r=0.9992 for GNP/SMCPE The
value of the slope of the obtained linear relations is less 0.5 which implies
that the electroactive species are transported by a diffusion process with an
adsorption contribution [36]. From the different investigated scan rates, the
100 mVs-1 gave the best voltammograms and higher selectivity. The
electrochemical oxidation peak potential (Ep) was also dependent on
the scan rate, where increasing the scan rate resulted in a shift to more positive
potentials, as shown in Figure 5 inset C. Ep(V)= 0.9046+0.0823 log ν(Vs-1)
r=0.9993 Ep(V)=0.8562+0.0819 log ν(Vs-1)
r=0.9996 In
order to determine the kinetic parameters of the electron-transfer process for
the DID oxidation on the GNP/SMCPE, Lavirons theory [37,38] for irreversible
processes was applied to calculate the number of electron transferred. E=Eo+2.303
RT/αnF[log RTKo/αnF]+2.303RT/αn F(log ν) Where,
R is the gas constant (8.314 J K mol-1), T is the temperature (298
K), F is the faraday constant (96485 Cmol-1), α is the electron
transfer coefficient, and n is the number of the electrons and αn can be
calculated from the slope of potential against log scan rate. In this system,
the slope were 0.0823 and 0.0819, αn
was calculated to be 0.719 and 0.720 for bare CPE and modified electrode
respectively, since for a totally irreversible electron transfer, α assumed as
0.5, then n was calculated to be 1.4 which indicated that one electron were
involved in the oxidation of DIN. Method
validation Linearity, LOD,
and LOQ: Under
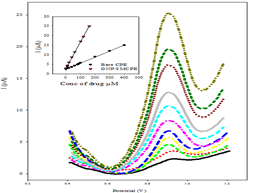
the above optimum conditions, the linearity using SWV was carried out where
good correlation between the oxidation peaks current (I) and concentration was
found in ranges of 2x10-5-4 x10-4 mol L-1, and
2x10-7-1.6x10-4 mol L-1 using bare CPE, and
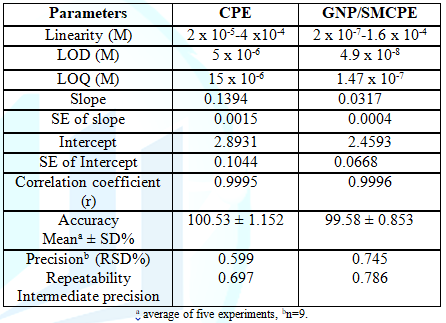
GNP/SMCPE (Figure 6). The Limits of Detection
(LOD) and the Limits of
Quantitation (LOQ) were calculated from the oxidation peak currents of the
linear ranges according to ICH guideline [39]. LOD and LOQ values confirmed the
sensitivity of GNP/SMCPE over bare CPE. The calibration equation parameters and
necessary validation data are shown in Table
1. Accuracy: The accuracy of
the proposed method for determination of DIN in drug substance was shown in
Table 1. The mean percentage recoveries were evaluated and satisfactory results
were obtained. The accuracy was further assessed by application of standard
addition technique (Table 2). Precision: The intraday and
interday precision were assessed by analyzing three concentration levels in
triplicate in a single assay run, and on three separate assay runs for the
drug, RSD% were less than 2%. This level of precision was adequate for the
quality control analysis of the drug as shown in Table 1. Robustness: The robustness of
the proposed method was demonstrated by constancy of the peak current with
deliberated minor changes in the experimental parameters. The studied variables
included; the change in pH (2.0 ± 0.2). These minor changes that may take place
during the experimental operation did not affect the peak current intensity of
the studied drug, indicating the reliability of the proposed method during
normal usage. Application
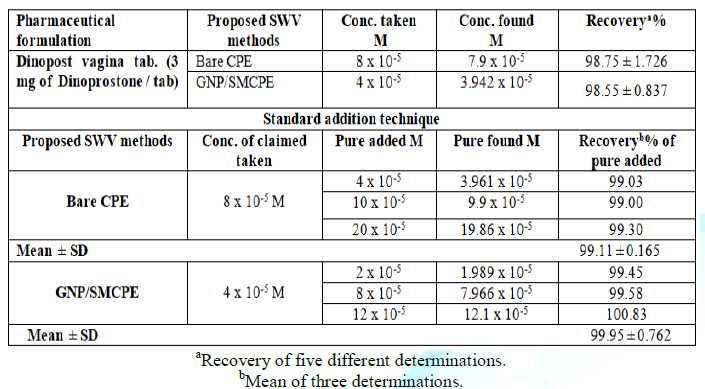
of the proposed SWV method for the determination of dinoprostone in
pharmaceutical preparation The
proposed SWV method was successfully applied to determine DIN in its pharmaceutical
formulation. The obtained results are listed in Table 2. The specificity of the proposed SWV voltammetric method
was proven by its ability to determine DIN in pharmaceutical formulation
without interference from excipients that commonly present. In
addition, the validity of the proposed SWV method was assessed by the standard
addition technique, Table 2. Statistical
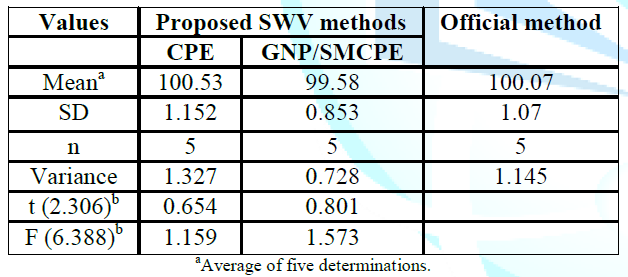
comparison between the results obtained by the proposed method and the official
method using student t-test and F ratio revealed no significant differences
with respect to accuracy and precision at probability 0.05% and the data was
presented in Table 3. Inexpensive
and eco-friendly SWV method was developed and validated for rapid sensitive
determination of DIN in drug substance and pharmaceutical dosage form.
The literature review revealed no attempted had been made for electrochemical
determination of DIN. The proposed method was based on the electrochemical
oxidation of DIN at both bare CPE and gold nanoparticle/silica-modified CPE as
a new fabricated sensor which causes an enhancement in the anodic peak current.
The results indicate the validity of the methods for application in routine
quality control, since it is characterized by high reproducibility and
selectivity. 1. Phillis
JW, Horrocks LA and Farooqui AA. Cycloxygenases, lipoxygenases, and
epoxygenases in CNS: Their role and involvement in neurological disorders
(2006) Brain Res Rev 52: 201-243. https://doi.org/10.1016/j.brainresrev.2006.02.002
2.
Bazan
NG, Colangelo V and Lukiw WJ. Prostaglandins and other lipid mediators in
Alzheimers disease (2002) Prostaglandins Other Lipid Mediat 68-69: 197-210. https://doi.org/10.1016/S0090-6980(02)00031-X
3.
Chen
C and Bazan NG. Lipid signaling: sleep, synaptic plasticity, and
neuroprotection (2005) Prostaglandins Other Lipid Mediat 77: 65-76. https://doi.org/10.1016/j.prostaglandins.2005.07.001 4.
Backlund
MG, Mann JR and Dubois RN. Mechanisms for the prevention of gastrointestinal
cancer: the role of prostaglandin E2 (2005) Oncology 69: 28-32. https://doi.org/10.1159/000086629 5.
United
States Pharmacopoeia (USP 40) (2017) United States Pharmacopoeia Convection,
Inc., Rockville, USA 6.
Pernoll
ML and Benson RC. Current obstetric and gynecologic diagnosis and management (6th
edn) (1987) CT: Appleton and Lange, Norwalk, USA 608. 7.
Reynolds
JEF. Martindale, the extra pharmacopeia (29th edn) (2011) London:
The Pharmaceutical Press, UK 1367. 8.
Christiansen
NJ, Bygdeman M, and Green K. Comparison of different prostaglandin analogues
and laminaria for pre-operative dilatation of the cervix in the late first
trimester abortion (1983) Contraception 27: 56-61. 9.
Lange
AP. Primary pulmonary hypertension during pregnancy: A case report(1983)
Acta Obstet Gynecol Scand Supp 113: 117-124. 10.
Yang
P, Felix E, Madden T, Fischer SM and Newman AR. Quantitative high-performance
liquid chromatography/electrospray ionization tandem mass spectrometric
analysis of 2- and 3-series prostaglandins in cultured tumor cells (2002) Anal
Biochem 308: 168-177. https://doi.org/10.1016/S0003-2697(02)00218-X 11.
Mohamed
MA, Atty SA, Salama NN and Banks CE. Highly Selective Sensing Platform
Utilizing Graphene Oxide and Multiwalled Carbon Nanotubes for the Sensitive
Determination of Tramadol in the Presence of Co‐Formulated Drugs (2017)
Electroanalysis 29: 1038-1048. https://doi.org/10.1002/elan.201600668 12.
Dryhurst
G and McAllister DL. Carbon electrodes, in laboratory techniques in
electroanalytical chemistry, Dryhurst G, McAllister DL, Kissinger P and
Heineman WR(Eds) (1984) Marcel Dekker Inc., USA. 13. Wang J.
Analytical electrochemistry (2nd edn) (2000) Wiley-VCH, USA. https://doi.org/10.1002/0471228230 14.
Mohamed
MA, Atty SA, Merey HA, Fattah TA, Foster CW, et al. Titanium nanoparticles
(TiO2)/graphene oxide nanosheets (GO): an electrochemical sensing platform for
the sensitive and simultaneous determination of benzocaine in the presence of
antipyrine (2017) Analyst 142: 3674-3679. 15.
Yang
RT. Adsorbent: fundamentals and application (1999) John Wiley, USA 131-134.
https://doi.org/10.1002/047144409X 16.
Keller
R. The Chemistry of Silica (1979) John Wiley, USA 896. 17.
Yantasee
W, Lin Y, Zemanian TS and Fryxell GE. Voltammetric detection of lead(II) and
mercury(II) using a carbon paste electrode modified with thiol self-assembled
monolayer on mesoporous silica (SAMMS) (2003) Analyst 128: 467-472. 18.
Sayen
S, Gerardin C, Rodehuser L and Walcarius A. Electrochemical Detection of Copper
(II) at an Electrode Modified by a Carnosine-Silica Hybrid Material (2003) Electroanalysis 15: 422-430. https://doi.org/10.1002/elan.200390049 19.
Yantasee
W, Lin Y, Fryxell GE and Busche BJ. Environmental Applications of
Nanomaterials: Synthesis, Sorbents and Sensors (2003) Anal Chim Acta 502:
207-212. 20.
Marino
G, Bergamini MF, Teixeira MFS and Cavalheiro Talanta ETG. Evaluation of carbon
paste electrode modified with organo-functionalized amorphous silica in for the
determination of cadmium(II) by using differential pulse anodic stripping
analysis (2003). 59: 1021-1028. 21.
Huang
W, Qian W, Jain PK and El-Sayed MA. The effect of plasmon field on the
coherent lattice phonon oscillation in electron-beam fabricated gold
nanoparticle pairs (2007) Nano Lett 7: 3227-3234. https://doi.org/10.1021/nl071813p 22.
Khatri
OP, Murase K and Sugimura H. Structural organization of gold nanoparticles onto
the ITO surface and its optical properties as a function of ensemble size
(2008) Langmuir 24: 3787-3793. 23.
De
Oliveira MK, Thaís dos Santos CC, Dinelli RL, Marinho ZJ, Lima CR, et al.
Aggregates of gold nanoparticles with complexes containing ruthenium as
modifiers in carbon paste electrodes (2013) Polyhedron 50: 410-417. 24.
Li
F, Song JX, Gao DM, Zhang QX, Han DX, et al. Simple and rapid voltammetric
determination of morphine at electrochemically pretreated glassy carbon
electrodes (2009) Talanta, 79: 845-850. https://doi.org/10.1016/j.talanta.2009.05.011 25.
Atta
NF, Galal A, Abu-Attia FM and Azab SM. Simultaneous determination of
paracetamol and neurotransmitters in biological fluids using a carbon paste
sensor modified with gold nanoparticles (2011) J Material Chem 21: 13015-13024.
26.
Atta
NF, Galal A and Azab SM. Novel sensor based on carbon paste/nation modified
with gold nanoparticles for the determination of glutathione (2012) Anal
Bioanal Chem 404: 1661-1672. 27.
Atta
NF, Galal A and Azab SM. Determination of morphine at gold
nanoparticles/Nafion® carbon paste modified sensor electrode Analyst (2011)
136: 4682-4681. https://pubs.rsc.org/en/content/articlelanding/2011/an/c1an15423k#!divAbstract 28.
Joseph
E Reynolds III, Josowicz M, Tyler P, Russell BV and Kyril MS. Spectral and
redox properties of the GFP synthetic chromophores as a function of pH in
buffered media (2013) Chem Commun 49: 7788-7790. 29.
Atta
NF, Galal A, Abu-Attia FM and Azab SM. Carbon Paste Gold Nanoparticles Sensor
for the Selective Determination of Dopamine in Buffered Solutions (2010) J
Elechtrochem Soc 157: F116-F123. 30.
Laviron
E. General expression of the linear potential sweep voltammogram in the case of
diffusionless electrochemical systems (1979) J Electroanal Chem 101: 19-28. https://doi.org/10.1016/S0022-0728(79)80075-3 31.
Smith
RJL and Masheder D. Amine oxidation. Part IX. The electrochemical oxidation of
some tertiary amines: the effect of structure on reactivity (1976) J Chem Soc
Perkin Trans 2 47-51. 32.
Siddappa
K, Mallikarjun M, Reddy T and Tambe M. Simple and Sensitive Extractive
Spectrophotometeric Method for the Assay of Mebeverine Hydrochloride in Pure
and Pharmaceutical Formulations (2008) J Chin Chem Soc 55: 1062-1068. https://doi.org/10.1002/jccs.200800155 33.
Hoh
GLK, Barlow DO, Chadwick AF, Lake DB and Sheeran SR. Hydrogen peroxide
oxidation of tertiary amines (1963) J Am Oil Chem Soc 40: 268-271. https://doi.org/10.1007/BF02633687 34.
Majidi
MR, Jouybanb A and Zeynali KA. Voltammetric behavior and determination of
isoniazid in pharmaceuticals by using overoxidized polypyrrole glassy carbon
modified electrode (2006) J Electroanal Chem 589: 32-37. https://doi.org/10.1016/j.jelechem.2006.01.016
35.
Atta
NF, Darwish SA, Khalil SE, and Galal A. Effect of surfactants on the
voltammetric response and determination of an antihypertensive drug (2007)
Talanta 72: 1438-1445. https://doi.org/10.1016/j.talanta.2007.01.053 36.
Gosser
DK. Cyclic voltammetry: simulation and analysis of reaction mechanism (1993)
Wiley-VCH, USA 156. 37.
Gushikem
Y and Rosatto SS. Metal Oxide Thin Films Grafted on Silica Gel Surfaces: Recent
Advances on the Analytical Application of these Materials (2001) J Braz Chem
Soc 12: 695-705. 38.
Bard
AJ and Faulkner LR. Electrochemical methods: fundamentals and applications
(2001) Wiley, USA 864. 39.
Validation
of analytical procedures: text and methodology Q2 (R1) (2005) International
conference on harmonization (ICH) harmonized tripartite guideline, Switzerland
1-13. Maha A Elabd, Department of Pharmaceutical Chemistry,
National Organization for Drug Control and Research, 6 Abou-Hazem st., Giza,
Egypt, E-mail: mahaelabd@hotmail.com
Atty SA, Walash M, Toubar S, AbouEl-Alamin MM, Elabd
EA, et al. Spot on gold nanoparticles/silica modified electrode for rapid
sensitive determination of dinoprostone (2019) Edelweiss Chem Sci J 2: 17-22 Dinoprostone, Square wave voltammetry, Sensor,
Carbon paste electrode, Gold nanoparticles/silica.Spot on Gold Nanoparticles-Silica Modified Electrode for Rapid Sensitive Determination of Dinoprostone
Shimaa A Atty,
Mohammed Walash, Safaa Toubar, Maha M AbouEl-Alamin,
Maha A Elabd and Nahla N Salama
Abstract
Full-Text
Introduction
Experimental
Results
and Discussion

Conclusion
References
Citation
Keywords