Research Article :
Here
we show that petroleum can be formed efficiently at normal temperatures and
pressures from carbon dioxide and activated water. The CO2 nano-bubble
containing water was treated with photocatalyst in the presence of oxygen under
UV irradiation. The activated water was mixed vigorously with kerosene or light
oil to form an emulsion. The emulsion gradually separated into a two-phase
solution. After phase separation, the volume of kerosene or light oil,
depending on which oil was utilized, increased by 5 to 10%. Likewise
tetradecane was used, and original tetradecane may be used as a template for
the synthesis of new tetradecane. When commercial light oil was treated in the
same way, most organic and inorganic impurities were removed from the oil and
very clean light oil was obtained. We named it as dream light oil. The dream
light oil meets strict Japanese Industrial Standards (JIS). Both
biotic and abiotic oil
hypotheses
have been proposed to explain the origin of oil on our planet. Supporting the
abiotic oil hypothesis, it has been known since 1967 that petroleum could be
formed at high temperatures and pressures from inorganic carbon in the form of
carbon dioxide with hydrogen or methane. The abiotic origin of petroleum has been
reviewed in detail by Glasby, who raises a number of objections, including that
there is no direct evidence to date of abiotic petroleum. Geologists now
consider the abiotic formation of petroleum scientifically unsupported, and
they agree that petroleum is formed from organic material [1]. However,
some argue that the abiotic theory cannot be dismissed because the mainstream
theory has yet to be established conclusively [2]. In 1920s, Fischer-Tropsch process was reported
indicating that petroleum can be produced from CO and H2 with
special catalyst under high pressures and high temperatures. Since then, no
apparent procedures on the topics have been exploited [3]. Here, we show that
petroleum can be formed efficiently at normal temperatures and pressures from
carbon dioxide and activated water. In addition, purification of commercial light oil (removal of
organic and inorganic impurities) is presented. Generation
of Activated Water Tap
water passed through reverse osmosis membrane (Toray
Industries, Inc., Osaka, Japan) to get pure water. Then CO2 gas was
supplied into the water by nano-bubble generator (Nishiken-devise, Osaka) for
30 min. The nano-bubbles are generally maintained in the water for a long
period of time unless CO2 is consumed. The nano-bubble containing
water was treated in the presence of oxygen gas with TiO2 catalysis (Takemoto
Industry, Okayama, Japan) under irradiation of UV light {UV sterilization lump: Panasonic GL-40
40w (254nm) and Black light: Toshiba FL40S BLB 40w (315nm-400nm, peak wave
length 352nm)} for 30min. Material
and quantity of photocatalyst is shown below. UV-reactive
TiO2 (70g), visible light-reactive TiO2 powder (70g)
(Ishihara Sangyo Kaisha, Ltd., Osaka, Japan), iron powder No. 300 (10g) (Wako Pure
Chemical Industries, Ltd., Osaka), platinum powder (1g) (Tokuriki Honten Co., Ltd.,
Tokyo), and 50g of acrylic resin adhesive (Bond FL200, Konishi Co., Ltd.,
Osaka) were suspended in 1liter of water glass (SiO2 28-30%, Na2O
9-10%, viscosity 100-250cp) (No.3, Fuji Kagaku Co, Osaka). This suspension was
mixed with ceramic support (1kg) and then dried. All
the powders adhered to the surface of ceramic support with acrylic adhesive. The 6kg of this
photocatalyst was set in the column. In this process, oxygen gas is converted
to ozone, and further to reactive oxygen species such as superoxide anion
radicals and hydroxyl radicals [4]. The
reactive oxygen species may reduce carbon dioxide to carbon monoxide, as follows, 2CO2
⇒ 2CO+O2 reaction (1) By
using TiO2 photocatalyst, H2O was decomposed into H2
and O2 as follows,
2H2O ⇒ 2H2+O2 reaction (2) As
a total,
CO2+H2O ⇒ CO+H2+O2
reaction (3) The
activated water (10L) containing reactive oxygen species was mixed vigorously
with either kerosene or light oil (10L) and carbon dioxide (from ambient air)
to form an emulsion by the special mixer (Takemoto Industry, Okayama, Japan). Analytical
method Alkane
was analyzed by column
chromatography
with SHIMAZU GC-2010. The experimental conditions are shown below. Vaporization room
for specimen Injection
mode: split Total flow rate: 13.0 ml/min Temperature
of vaporization room: 250oC Column
flow rate: 1.81 ml/min Carrier
gas: He Linear velocity: 40.0 cm/sec Control
mode: linear velocity Purge
flow rate: 4.0 ml/min Pressure:
142.0 kPa Split ratio: 4.0 Column oven: Column oven
temperature program: initial 75oC, Rate:
6oC/min, Final 300oC, Hold 20 min Column:
DB-1 (0.25μm × 30m) (J and W Scientific, California) Detector Detector:
FID, Make up flow rate: 30.0 ml/min Detector
temperature: 300oC, Flow rate of H2: 47.0 ml/min Make
up gas: He, Air flow rate: 400.0 ml/min The
inorganic impurities from commercial light were analyzed by X-ray Fluorescence. Chemical
Synthesis of Oil Ten
liter of light oil and activated water (10 liter) were collided against the
wall of the mixer. The white emulsion (Figure 1) was left static until it
separated into a two-phase (oil-water) solution (Figure 2). The volume of the oil fraction increased from the
original volume by 5 to 10% and water fraction decreased by 5 to 10%. All
reactions were carried out at room temperature and normal pressure. The
oil generation reaction may occur as radical polymerization in emulsion and
be written as follows, nCO+(2n+1)H2
⇒ CnH2n+2+nH2O
reaction (4) This
reaction is a part of Fischer-Tropsch process [5-7]. Generally,
the Fischer-Tropsch process is operated in the temperature range of 150-300oC.
Typical pressures range from one to several tens of atmospheres. Converting a
mixture of H2 and CO into aliphatic products obviously should be a
multi-step reaction with several sorts of intermediates, 1) associative adsorption of CO, 2)
splitting of the C/O-bond, 3) dissociative adsorption of 2H2, 4)
transfer of 2H to the oxygen to yield H2O, 5) desorption of H2O,
6) transfer of 2H to the carbon to yield CH2. These processes are
repeated as radical polymerization for the growth of the hydrocarbon chain. Why such
intermediates are not detected in the present reaction will require further
in-depth analysis. From reactions 3 and 4, mass balance is shown as follows, nCO2+(n+1)H2⇒
CnH2n+2+nO2 reaction (5) Comparison
between original light oil and new oil: The original oil and new oil (a mixture
of original oil and newly synthesized oil) were analyzed by chromatography.
SHIMAZU GC-2010 was used for the chromatographic analyses. Distribution of
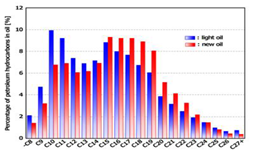
carbon number of original light oil was compared with that of new oil (Figure 3). Difference in compositional
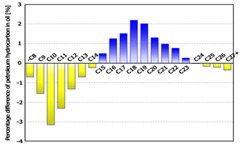
ratio at each carbon number was compared in Figure 4. Hydrocarbons with 15 (C15) to 23 (C23)
carbon atoms increased in the new oil, whereas C8 (or less) through
C14 decreased and C24 through C27 (or more)
decreased. These data clearly show that C15 through C23
was synthesized more efficiently than the original light oil and the lower
number and higher number hydrocarbon were synthesized less. If all the
fractions (C8 through C27) were synthesized at the same
ratio, the difference of new oil and original oil should be zero. Thus we can
conclude the chemical
synthesis of hydrocarbon from CO2 and water. Figure 3: Comparison of
composition between original light oil and new oil. Characteristics
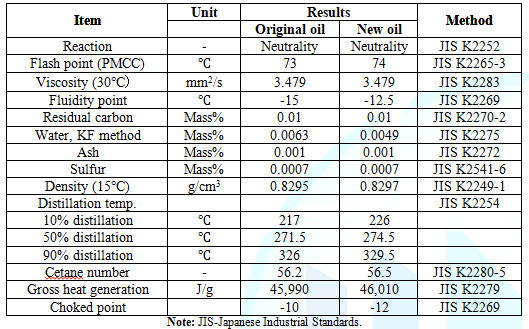
of newly generated oil and the original oil are compared as shown in Table 1. No apparent difference between
the two oil samples was recognized. A slightly higher value of gross heat
generation in new oil may reflect the different distribution of carbon number. Figure 4: Difference in
compositional ratio at each carbon number between original oil and new oil. Table 1: Characteristics
of original and new oils. Production
of Specific Carbon Number n-Alkane In order to examine the possibility of
producing oil with a specific carbon number, the special grade of tetradecane
(C14) was purchased from Wako Pure Chemicals (Osaka, Japan)
and used as the original oil for this reaction. The volume of new tetradecane
increased by 7%. The chromatographic data are shown in Figure 5a, Figure 5b and Figure 5c. Figure
5a shows the chromatogram of hexane (C6) used as the solvent. Figure
5b shows the chromatogram of the original tetradecane. Figure 5a: Chromatogram of
hexane as solvent. Figure 5b: Chromatogram of
original tetradecane Figure 5c: Chromatogram of
newly synthesized material from tetradecane. The
original sample of tetradecane is composed of 0.021% tridecane (C13),
99.804% C14, and 0.175% pentadecane (C15). The new oil
contained 0.023% C13, 99.793% C14, and 0.184% C15.
Although the oil volume increased, no apparent difference of chromatographic
data was observed. Original tetradecane may be used as a template for the
synthesis of new tetradecane. These data show that specific carbon number hydrocarbon
(alkane) could be synthesized depending on the original alkane. Therefore it is
easily understood that light oil was produced with light oil and kerosene was
synthesized with kerosene. Purification
of Commercial Light Oil Commercial
light oil was purchased from gasoline stand, and treated likewise. The volume
of oil was increased by 5-10% as mentioned above. Surprisingly after the
reaction, organic soot
fraction
(impurity from commercial light oil) appeared in the water fraction at oil-water interface (Figure 6). At the same time, inorganic impurities appeared at the
bottom (Figure 7). The inorganic
impurities include mainly Fe, Si, Zn, and a little amount of K, S etc. Figure 6: Soot fraction
from commercial light oil. Figure 7: Inorganic
impurities from commercial light oil. Color
of commercial light oil and newly synthesized oil was compared (Figure 8). Commercial light oil (left)
showed dark yellow and new oil (right) showed pale yellow, because organic and inorganic
impurities were removed from commercial light oil. When this clean new oil was
repeatedly used for the next process, new clean oil was produced, indicating
inexhaustible synthesis. Figure
8: Comparison of commercial light oil (left) and new
oil (right). Application
of New Oil Since
newly synthesized clean light oil meets strict Japanese Industrial Standards (Table 1), the
performance was examined by using diesel engine. In fact, one 10 ton truck, two
4 ton trucks, and a sedan car were used. The results were very good. (1)
Traveling distance was extended by 1.15 ~ 1.50 fold. (2) Exhaust gas became
clear. Amount of soot decreased drastically. (3) Knocking was hardly
experienced, indicating anti-knocking. (4) Engine sound was getting more quiet.
Since the clean light oil is excellent, it was named as Dream Light Oil. Petroleum (light oil,
kerosene, n-alkane) could be formed efficiently at normal temperatures and
pressures from carbon dioxide and activated water. The CO2 nano-bubble
containing water was treated with photocatalyst in the presence of oxygen under
UV irradiation. The activated
water was mixed vigorously with kerosene or light oil to form an emulsion. The emulsion
gradually separated into a two-phase solution. After phase separation, the
volume of kerosene or light oil, depending on which oil was utilized, increased
by 5 to 10%. Likewise, tetradecane was used as a template in the experiment,
only tetradecane was newly synthesized. When commercial light oil was treated
in the same way, most organic and inorganic impurities were removed from oil
and very clean light oil was obtained. We named it as dream light oil. The
dream light oil meets strict Japanese Industrial Standards. The oil showed very
excellent performance for diesel engine. Since
this oil production process is cheap and effective, this technique is extremely
important to deal with the current energy crisis, and should provide a
foundation for carbon-neutral energy production. The method simultaneously
utilizes/reduces carbon dioxide (greenhouse gas) as it is
consumed as a carbon source when converted to hydrocarbons. Since the newly
synthesized oil does not contain sulfur, SOx is not exhausted from
the combustion gas. This result may also support the abiotic petroleum origin
hypothesis. These
results can be summarized as follows, (1) Fuel hydrocarbon can be synthesized
efficiently at normal temperatures and pressures from carbon dioxide and
activated water. (Inexhaustible resources), (2) Gross heat generation of
original oil and new oil were the same, (3) High productivity, (4) High cost
performance (Efficient process), (5) Oil is used as a template for next oil
synthesis, (6) No need to purify the new oil, because S and N are not contained
in the dream oil. Thus, SOx and NOx are not generated
after burning (Earth friendly), (7) Soot fraction and other impurities were
removed from commercial light oil, and the new dream oil showed excellent
performance for diesel engine, (8) Since CO2 in the air is mainly
consumed, this process contributes to global warming prevention. (Emissions
trading is advantageous), (9) Carbon neutral, (10) Abiotic synthesis of
petroleum, (11) Energy crisis will be avoided. 1.
Glasby
GP. Abiogenic origin of hydrocarbons: an historical overview (2006) Resour Geol
56: 83-96. http://dx.doi.org/10.1111/j.1751-3928.2006.tb00271.x 2.
Speight
JG. The chemistry and technology of petroleum (2006) CRC Press, United States. 3.
Fischer-Tropsch
process, Wikipedia, the free Encyclopedia (2015). 4.
Ono
R, Nakagawa Y, Tokumitsu Y, Matsumoto H and Oda T. Effect of humidity on the
production of ozone and other radicals by low-pressure mercury lamps (2014) J Photochem
Photobiol: Chem 274: 13-19. https://doi.org/10.1016/j.jphotochem.2013.09.012 5.
Schulz
H. Short history and present trends of Fischer-Tropsch synthesis (1999) Applied
Catalysis A: General 186: 3-12. https://doi.org/10.1016/S0926-860X(99)00160-X 6.
Kaneko
T, Derbyshire F, Makino E, Gray D and Tamura M. Coal liquefaction (2001) Ullmanns
Encyclopedia of Industrial Chemistry, Wiley-VCH, Germany. https://doi.org/10.1002/14356007.a07_197 7.
Khodakov
AY, Chu W and Fongarland P. Advances in the Development of Novel Cobalt Fischer-Tropsch
Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels (2007)
Chemical Reviews 107: 1692-1744. https://doi.org/10.1002/chin.200733255 Tadayuki Imanaka, The Research Organization of
Science and Technology, Ritsumeikan University, 1-1-1 Noji-Higashi, Kusatsu,
Shiga, 525-8577, Japan, E-mail: imanaka@sk.ritsumei.ac.jp
Imanaka T and Takemoto T. Chemical synthesis of fuel
hydrocarbon from CO2 and activated water, and purification of
commercial light oil for dream oil (2019) Edelweiss Chem Sci J 2: 23-26. Fuel hydrocarbon production, CO2,
Activated water, Photocatalyst, Purification of commercial oil.Chemical Synthesis of Fuel Hydrocarbon from CO2 and Activated Water, and Purification of Commercial Light Oil for Dream Oil
Tadayuki Imanaka and
Tadashi Takemoto
Abstract
Full-Text
Introduction
Materials
and Methods
Results
and Discussion








Conclusion
References
*Corresponding author
Citation
Keywords