Editorial :
Takashiro Akitsu*,
Yuika Onami and Natsuki Katsuumi One of the most probable and frequent reasons
for a chemical experiment fire accident may be dangerous heating as well as the
reagent itself (e.g. volatility and ignition) during a chemical reaction. Some
reaction methods without heating (such as long-time stirring at room
temperature) and using advanced tools (such as a microfluidic device), which
are associated with unusual intermediates kinetically and may result in
yielding unexpected products thermodynamically. This paper focused on two
complicated examples of crystal structure analysis of unexpected and/but normal
products. Generally, heating during chemical reactions are
common procedure to enhance reaction rate, though it may be common reason for
fire accidents in chemical laboratory [1]. Not only for avoiding fire but also
for obtaining products efficiently, alternative experimental conditions or
methods can be selected at room temperature. One may be long-time reaction can
help slow rate not to exceed activation energy to become usual intermediate
kinetically. Another may be using sophisticated tools such as a microfluidic
device, in which moving solvents, encounter of the reactant can be caused in a
short time. Additionally, limited special environment may result in formation
of unusual products thermodynamically [2]. In recent years, we have studied on preparation of
(chiral) Schiff base metal complexes (Scheme
1) in hot alcohol solutions for several hours [3]. As for copper(II)
complexes having chelate Schiff base ligands, typical reaction scheme can be
described as Scheme 1. Scheme1:Typical reaction scheme of metal complexes having (chiral) Schiff base ligands However, some unusual reactions yielded products of
single crystals, of which structure analysis was complicated because there was
also possibility of unexpected products. According to such working hypothesis,
two strange examples after reactions of failed conditions found in our group
are introduced below. Case
1: “Excess” hydrate of copper(II) acetate, (Cu(COOCH3)2.H2O).0.5H2O As recovery of the reactant (namely, no reaction
occurred), copper(II) acetate hydrate as a metal source was found by X-ray crystallography.
However, its cell constants and composition (containing 0.5 crystalline water)
were different from the original and re-determination reports. Adsorption of
crystalline solvents under different reaction conditions was expected firstly [4].
Due to too large thermal displacement parameters for 0.5 H2O,
re-calculation after removing weak diffraction data in reciprocal space were
carried out (We soon realized the mistake, so we didn't leave any wrong
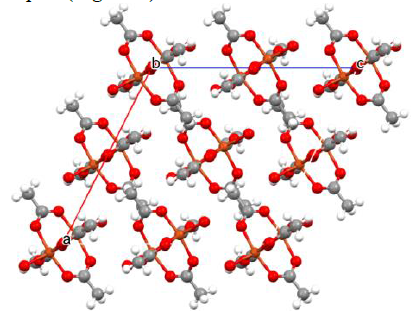
results.). Then it was solved as the following unit cell data: C2/c, a=13.192(9), b=8.595(6), c=13.867(10)Å, β=116.913(11)°, V=1402.0(18)
Å, being identical to the original report (Figure
1). Case
2: Not monoclinic but “triclinic” polymorphism of 2-hydroxy-1-naphthaldehyde (C11H8O2) As recovery of the reactant, a single crystal of
2-hydroxy-1-naphthaldehyde (instead of salicylaldehyde, o-C6H5(OH)(CHO)=
C11H8O2) was obtained and its crystal
structure was determined at low temperature (173K). An analysis program
automatically judged to be triclinic
P-1 with Z=4 based on unit cell constants (a=5.626(2),
b= 9.360(4), c=15.522(7)Å, α=90.027(5),
β= 80.695(6), γ= 90.087(6)°, V=806.7(6)
Å. It cannot be negligible deviation of α
and γ from exact 90 degrees according
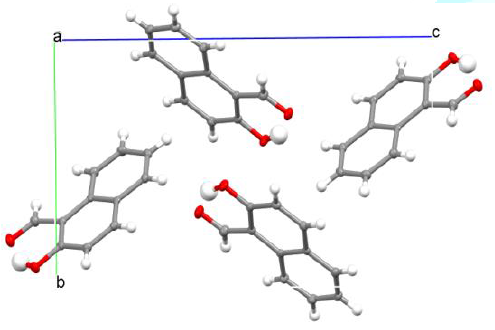
to standard deviation reasonably. Two crystallographically independent
molecules in asymmetric unit were perfectly identical, which suggest n-glide
plane as a proof of monoclinic P21-/n
being similar to the original report measured at room temperature [5].
Therefore, we judged again systematic absence (0k0) manually and confirmed
crystallographic symmetry of unit cells, namely, Laue group 2/m (of course,
monoclinic crystal system) based on both 21 screw axis and n-glide
plane. In this way, it was not polymorphism (same compounds taking different
crystal structures) by phase transition but wrong determination of crystal
system (Figure 2). In the two examples, humans corrected the
crystallographic mistakes by the programs, but it can also be interpreted that
the reaction conditions produced “subtle” products. In other word, this area is
suspicious. Indeed, novel methods of chemical reaction or special condition of
crystallization may result in thermodynamically unexpected products or unusual
isolation of kinetically favored products, novel asymmetric synthesis of
optical isomer (or racemization), unstable tautomer, chiral crystallization,
new phase (polymorphism) of crystals, inclusion of guest molecules and so on,
respectively. However, careful experiments must be also required to discuss
such “delicate” chemistry. This work was supported by a Grant-in-Aid for
Scientific Research (A) KAKENHI (20H00336). Takashiro Akitsu, Department of
Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku,
Tokyo 162-8601, Japan, E-mail: akitsu2@rs.tus.ac.jp
Do Thermally Mild Chemical Reactions (for Avoiding Fire Accidents) Give Rise to Unexpected Products?
Abstract
Full-Text
Introduction

Results
and Discussion
Conclusion
Acknowledgement
References
Corresponding
author
Citation
Akitsu T, Onami Y and Katsuumi N. Do
thermally mild chemical reactions (for avoiding fire accidents) give rise to
unexpected products? (2020) Edelweiss Chem Sci J 3: 15-16.
Chemical reaction, Temperature, Schiff base
metal complexes, Unexpected products, Crystal structure analysis.