Research Article :
Topwe Milongwe Mwene-Mbeja Beer can be considered as a hydrous solution of
ethanol, in which several organic substances are dissolved. These organic
substances are subject to a variety of reactions, which occur during
fermentation, storage, and eventually these kinds of chemical reactions
determine the characteristic aging of the beer, and its quality as well.
Beer
is obtained by fermentation of glucose molecules of plant origin. In other
words, beer is an alcoholic beverage obtained by transformation of starchy
substances by enzymatic and microbiological means. The aim of this review is to
show different organic reactions, which take place during the production of
beer with emphasis on the reactivity of organic compounds as well as the
reaction mechanisms. It is important to mention that the understanding of a
reaction mechanism helps to select starting materials and predict the formation
of a desired product or the knowledge of a reaction mechanism permits to set up
appropriate conditions in order to generate a targeted product. Water Water
in the beer must be free of organic and inorganic pollutants or any undesirable
products such as bacteria, sediments, halogenated aromatic organic compounds. Water
contains mineral salts, which must be controlled. Water is necessary for
brewing as well as for cleaning and rinsing equipment used in the brewery.
Spring or well water is desirable because it contains a small amount of mineral
salts, but it has to be checked regularly in order to verify its purity. It is
very important to mention that the quality of beer depends on the chemical
characteristics of water [1,2,3]. Malt Malt
is made from cereals particularly barley, which is most often utilized in
brewery. In this regard, at the malt house, cereals are placed in conditions
which allow their germination and then, they are dried. This kind of technology
is called malting, a technology that sets free enzymes, which hydrolyse the
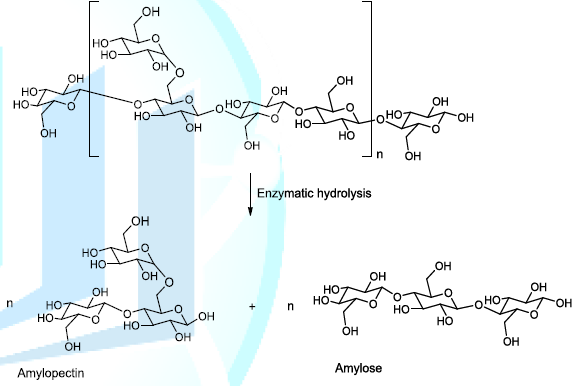
starch to produce amylopectin (branched polymer of glucose molecules) and
amylose (linear chain composed of glucose molecules) into the reaction medium (Scheme 1). The plausible enzymatic
mechanism of starch hydrolysis has been recently reported in the literature [4,5]. Yeasts Yeasts
are microscopic unicellular fungi that live and proliferate by consuming sugars.
In anaerobic conditions, yeasts convert sugars into ethanol and carbon dioxide.
This kind of conversion is called fermentation. This chemical process is the
basis for the production of any alcoholic beverage. During fermentation, the
yeasts also produce a variety of aromatic substances, which give the beer its
own characteristic [5,6]. Caramelization Caramelization
is a non-enzymatic oxidation reaction extensively utilised in cooking to obtain
the natty flavor and brown colour. During caramelization, volatile organic
substances are released, and that allows a characteristic flavor of the
caramel. Indeed the reaction involves the loss of water in the form of vapor,
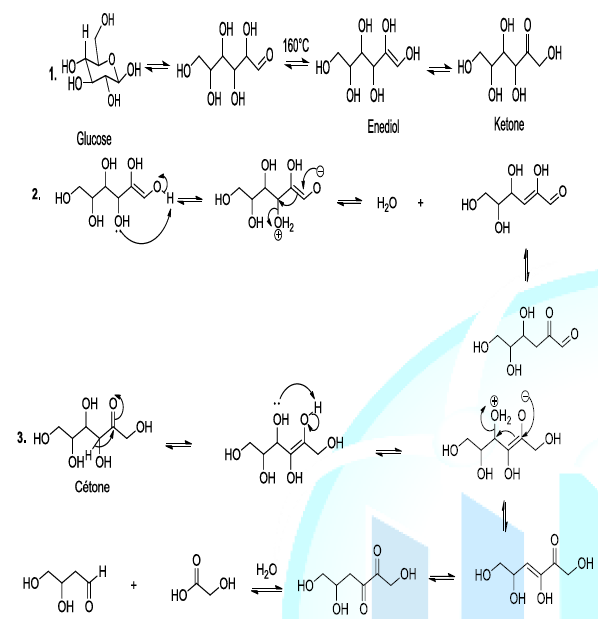
and the cleavage of glucose (Scheme 2,
reaction 1). The thermal degradation of sugar, during the caramelization
process, involves diverse reactions such as the intra molecular rearrangement
or Lobry-de Bruyn-van Ekenstein Rearrangement. This step is followed by beta
elimination of a molecule of water (dehydration reaction) and dicarbonyl group
cleavage as well (Scheme 2, reaction 2,3)
[7-10]. Scheme 2: Thermal decomposition of glucose Most
importantly, the enolization of glucose is a pivot reaction because it
initiates the degradation process. Indeed, the organic compounds generated
during the thermal decomposition of glucose can subsequently react to produce
carboxylic compounds, and oxygenated heterocyclic substances via aldol
condensation. From all the sugar degradation reactions, the strategic
intermediate compounds from thermal caramelization are dicarbonyl compounds.
These kind of compounds lead not only to the formation of caramel coloration,
but also they generate volatile products, which are typical of the caramel
flavor [7-10]. Humulus
lupulus Hop
or Humulus lupulus L. is a climbing plant is the Cannabaceae family. The female
plants produce flowers in the form of small cones made up of leaflets.
Underneath these small leaves are tiny yellow glands with lupulin, which
contains better resins and aromatic essential oils. Therefore, the hop gives
the beer a bitter taste and a characteristic aroma according to the used
technology. It contributes to the retention of foam, and to the shelf life of
the beer. It can be utilized as a cone, its coolest form or as a compressed
granule, which is practical and durable. In addition, the tannic substances
contained in the leaflets allow the hop to play the role of preservative and
natural clarifier of the beer [11-13]. During the beer production, lupulin is
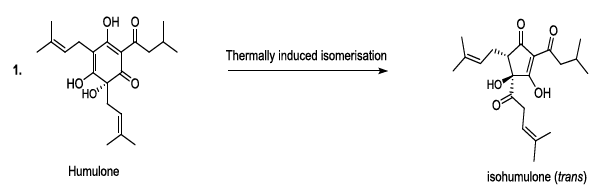
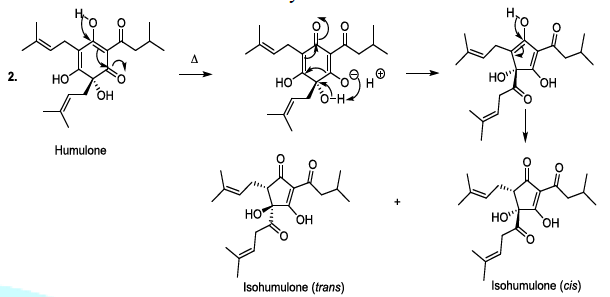
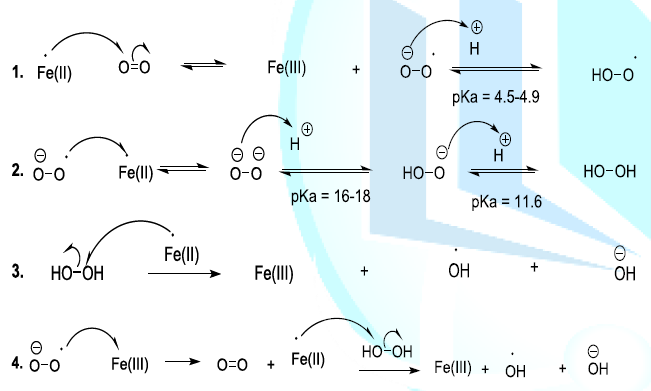
transformed to produce bioactive humulone and lupulone (Scheme 3). The antioxidant and antibacterial Lupulone as well as
humulone both participate to the preservation of beer. Under thermal
conditions, Humulone is, at its turn, converted to isohumulone, an
antibacterial with a bitter flavor (Scheme 3) [14-22]. Scheme 3a: Thermally induced isomerisation Scheme 3b: Thermally induced isomerisation Scheme 4: Isohumulone degradation mechanism The
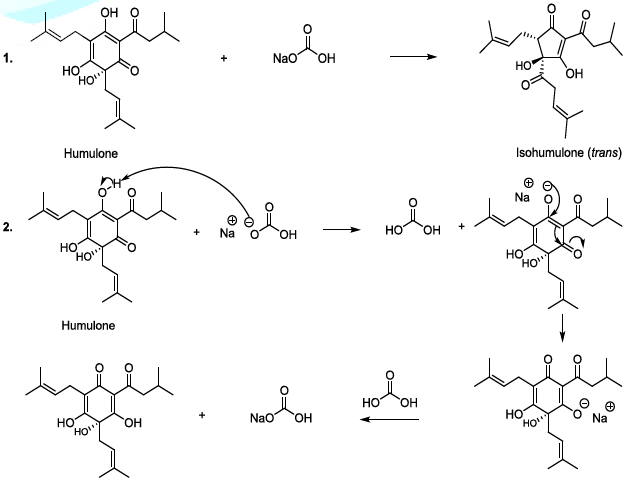
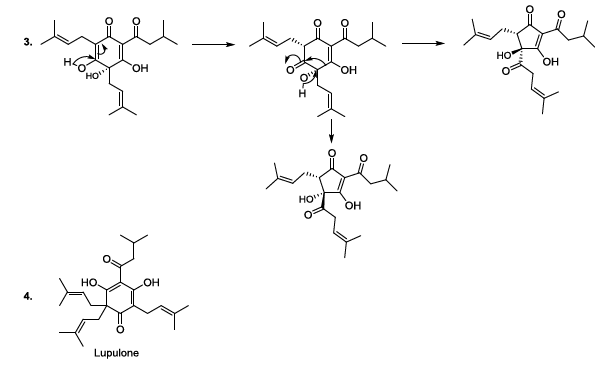
isomerization of humulone can also be carried out in mild alkaline conditions,
and in this case, the plausible mechanism involves the formation of a single
anion (Scheme 5). This sequence is
followed by the formation of a ketone group in stereospecific manner, and the
cyclic contraction to furnish trans and cis isohumulone in ratio of 32/68
[14-22]. It has been reported that in harsh alkaline conditions, two anions are
formed, and this results in the production of two trans and cis isohumulone in
ratio of 50/50 [14-22]. Scheme 5: Isomerization of humulone mechanism Oxygen Oxygen
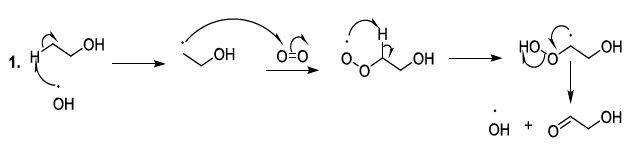
alters rapidly the flavorof the beer. This means that oxygen is the initiator
of chemical reactions that produce oxygenated reactive entities. Indeed, Oxygen
in the ground state is normally stable and it cannot easily react with organic
compounds. Nevertheless, in the presence of Fe(II) or Cu(I) in beer, the oxygen
can capture an electron to form a radical anion, which can remove a proton to
generate a more reactive hydroxyl radical. This later can also react with iron
(II) or copper (I) to produce a peroxide anion. In beer, the peroxide anion is
transformed into hydrogen peroxide. The hydroxyl radical can be produced from
the hydrogen peroxide (Fenton reaction) or from oxygen (radical anion) by
metallic induction (Scheme 6, reaction 1-4)
(Haber-Weiss reaction) [23-26]. Scheme 6: Generation of oxygenated reactive entities Oxygenated
entities degrade organic constituents of beer, and correspondingly, they
promote the aging or deterioration of the beer quality (Scheme 7) [23-26]. Scheme 7a: Degradation of organic constituents of beer Scheme 7b: Degradation of organic constituents of beer mechanism Fatty
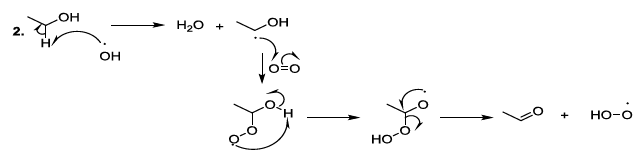
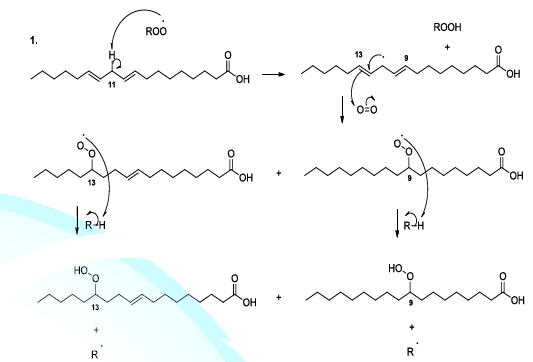
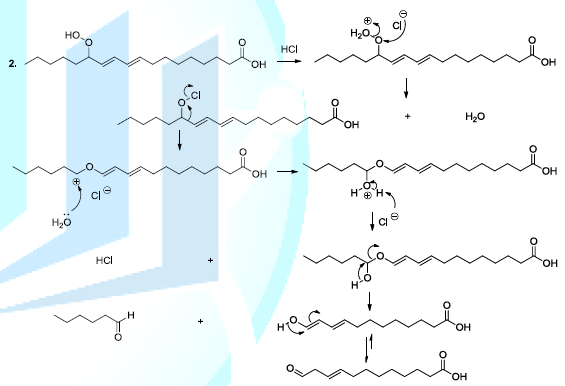
acids Reactive
oxygenated species also react with lipids or fatty acids. Indeed, the oxidation
of lipids starts with the removal of a hydrogen atom by free radicals specially
hydroxyl radicals or peroxides. In the case of linoleic acid, the hydrogen
situated upon carbon 11 is a hydrogen atom that is easy to be removed because
it is activated by the two neighboring double bonds. It has been reported that
hydroperoxide acids can be decomposed, in acidic conditions, to produce diverse
volatile compounds, and in this perspective, several reaction mechanisms have
been proposed whose the ionic mechanism (Scheme
8, reactions 1,2) [23-26]. Scheme 8a: Fatty acid oxidation mechanism
Scheme 8b: Fatty acid oxidation mechanism Carbonyl
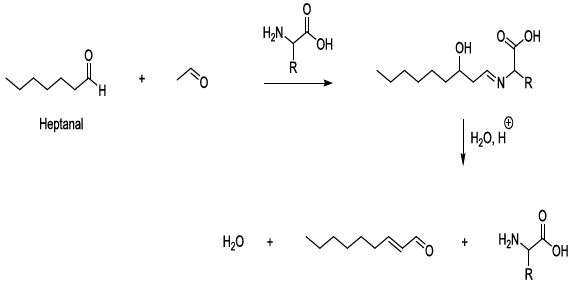
compounds Experimental
observations stated that aldol condensation of carbonyl compounds is plausible
under mild conditions in beer during storage, and in this regard, amino acids
behave as bases, nucleophiles, organic catalysts, and they contribute to the
formation of imine intermediate products. This kind of process can lead to the
production of carbonyl compounds after the hydrolysis of the corresponding
imines (Scheme 9) [23-26]. Scheme 9: Catalysis of aldol condensation This
review has shown enzymatic and non-enzymatic organic reactions occurring in
beer production in order to better understand, for example, why oxygen has to
be prevented to get in contact with beer. This is very important so that the
quality of beer remains optimal. The knowledge of organic reactions also helps
to understand the importance of preventing oxygen during fermentation because
it will destroy the enzymatic transformation of sugars due to the presence of
yeasts in the production of alcoholic beverage. Organic reactions play a
notable role because the aging of beer can be preserved in using additives such
as ascorbic acid. In fact, beyond the technology of measurements on devices to
reduce the influence of atmospheric oxygen during bottling, ascorbic acid has
proven to be a suitable oxygen reducer or a reliable antioxidant during beer
brewing. Chemistry, Organic Compound, Mechanism and BeerChemistry of Organic Compounds in the Beer Production
Abstract
Full-Text
Introduction
Chemical
Ingredients

Conclusion
References
Corresponding author
Topwe Milongwe
Mwene-Mbeja, Department of Chemistry, Faculty of Science, University of
Lubumbashi, Lubumbashi, Democratic Republic of the Congo, Hydro-Quebec
Institute for Environment, Development and Society at Laval University, Quebec,
Canada, Email: topwe.mwenembeja@unilu.ac.cd; topwe@hotmail.ca
Citation
Mwene-Mbeja TM. Chemistry of organic compounds in the beer
production (2020) Edelweiss Food Sci Tech 1: 32-35. Keywords