Case Report :
Mario
Giordano, Gianpiero Gaio, Maurizio
Cappelli Bigazzi andMaria Giovanna Russo Percutaneous stenting of the pulmonary artery is
a diffuse procedure, above all in patients with congenital heart disease. The
effectiveness of the procedure is associated with potential and feared
complications. This case describes the embolization of a premounted stent into
the left lower lobe pulmonary artery in a 3-years old patient with
univentricular heart and left-side superior vena cava undergone to Norwood
procedure modified according to Sano (stage I) and then bilateral
cavo-pulmonary anastomosis according to Glenn (stage II). The retrieval
procedures were complicated by the severe hypoplasia of the left pulmonary
artery and by the complex anatomy of the patient. This case shows how to
pull-back an embolized stent in a child with a complex congenital heart disease
despite the limitations related to the low weight and the complex vascular
anatomy. Pulmonary artery and its branches
stenting is an effective and diffused procedure to treat severe stenosis of
these arterial vessels. Stenosis suitable for percutaneous stenting is which
occurs due to: kinking or tension external compression intimal flaps surgical
intervention or developed after a balloon angioplasty [1].
In a multicenter study conducted
by van Gameren et al., pulmonary artery stenting–related complications were common
(17%), and stent malposition/migration/embolization was the major complication
(49%) [2]. Although several case reports, observational studies, metanalysis
and reviews were published about the retrieval of a migrated or embolized stent
during the percutaneous coronary intervention [3-5] only a few cases about
stent dislocation in children or in patients with congenital heart disease are
described. Ashwath et al. published a series of 15 patients weighting less than
10 kilograms underwent percutaneous stenting with a case of a stent embolized
to the distal left pulmonary artery and successfully recaptured, although it is
not explained the technique used to pull-back the stent [6]. The aim of this
case report is to describe the difficulties and the complications associated to
percutaneous stenting of pulmonary artery in a child with complex anatomy and
the technique used to solve the complications developed. M.S. is a male child with fetal
diagnosis of double outlet right ventricle, mitral valve atresia and
persistence of left-side superior vena cava. At 7 days of life, he underwent
Norwood procedure (stage I) modified according to Sano, whereas at 7 months, he
was subjected to bilateral cavopulmonary anastomosis according to Glenn (stage
II) plus enlargement of the left pulmonary artery with a pericardial patch. He
was followed periodically through cardiologic visit, electrocardiogram, and
echocardiography. At the age of 3 years old (weight 11 kg and height 120 cm),
the echocardiography showed no evidence of the left pulmonary artery. For this
reason, it was decided to evaluate the caliper of pulmonary arteries through a cardiac
catheterization. Clinical examination showed: a sisto-diastolic murmur on
second left intercostal space, jugular vein distension and signs of central
cyanosis. Pulse-oximetry saturation was 80% (FiO2: 21%). Echocardiography
demonstrated an adequate systolic function of systemic right ventricle
(tricuspid annular plane systolic excursion: 19 mm) and a mild regurgitation of
tricuspid valve. From a venous vascular access (right internal jugular vein), a
6 Fr pig-tail catheter was brought to the pulmonary bifurcation side. Pulmonary
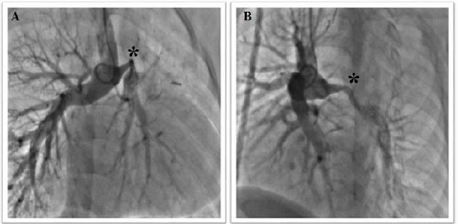
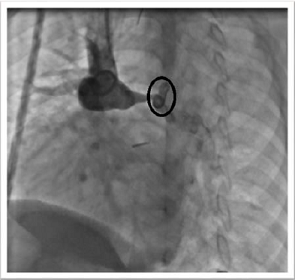
angiography showed a long severe stenosis of the left pulmonary artery (length
26 mm and diameter 2 mm, z-score -8, 54) at the site of the left-side superior
vena cava anastomosis (Figure 1).
At hilum side, the left pulmonary
artery was measured around 8 mm. The lesion was crossed by a 0.014” wire, and
then a more supportive wire (Amplatzer super stiff 0.035”) was placed into the
left inferior lobar artery. It was chosen to implant a stainless steel,
pre-mounted, open-cell stent: Valeo® balloon expandable vascular stent 36 x 8
mm (Bard Peripheral Vascular, Tempe, Arizona, United States of America), to
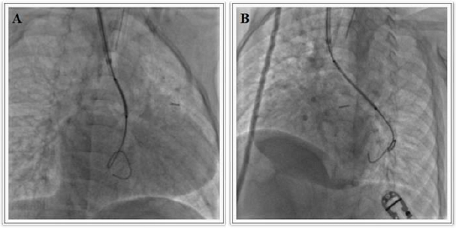
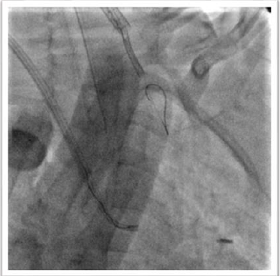
cover the left pulmonary artery completely. When the stent was brought into the
stenotic segment, it seemed to be too much long since it protruded into the
inferior lobar artery (Figure 2) and
for this reason, it was decided to pull-back its. Nevertheless, despite a large
delivery system (Mullins sheath 8 Fr), during the retrieval, the stent slipped
from the balloon by positioning beyond the closer segment of the pulmonary artery and by engaging the left inferior lobar artery (Figure 3). The loss stent was crossed by a
0.014” wire and a progressive inflation of small coronary balloons (Tazuna®
semi-compliant balloon catheter 1.5 x 20 mm, 2.0 x 20 mm and 2.25 x 20 mm) was
achieved to anchor and pull-back the stent. The balloons were inflated until to
achieve a pressure of 10-12 atm (rated burst pressure of the balloon: 14 atm).
No balloon bursted during these manoeuvres. This technique of retrieval could
expose the patient to a high risk of vessel dissection, but in this case the
stenotic tract was a fibrous cord with a great endurance to parietal stress and
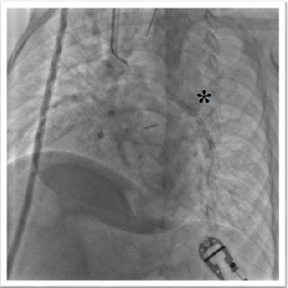
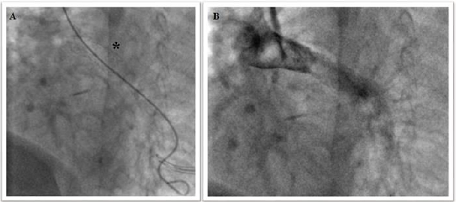
for this reason the dissection was avoided despite so rough margins. The retrieval of stent failed since it wasnt
able to cross the narrowest tract of pulmonary artery (Figure 4). Luckily, the end-to-side anastomosis of the left-side
superior vena cava to the left pulmonary artery was beyond the narrowest tract
(Figure 5). It was decided to achieve one
more vascular access on the left side (left internal jugular vein) and to
insert a large introducer (11 Fr) to pull-back the stent into itself. The
various inflation and deflation of different balloons had caused a progressive
enlargement of proximal-end of the stent and the balloon wasnt able to anchor
the stent for this reason, it was used an Amplatz GooseNeck® Snare to tighten
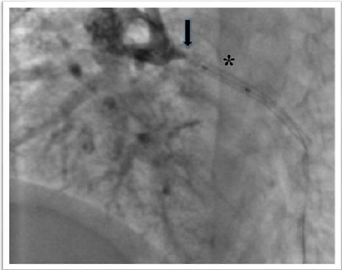
the enlarged tract of the stent and to allow the anchor of the balloon. The
stent was adequately pulled-back into the introducer that was removed with half
stent into and half stent outside of itself (Figure 6). An adequate haemostasis was achieved through a manual
compression of left internal jugular vein. Despite the complication, by using
the right-side vascular access, the stenosis was crossed by a wire and a
pre-mounted Valeo® balloon expandable vascular stent 26 x 8 mm was implanted
and post-dilated until a diameter of 10 mm, with a good angiographic result (Figure 7). Final pulmonary angiography
showed a balanced flow through both pulmonary arteries and no signs of
dissection or thrombo-embolic complications. In the catheterization laboratory,
a dose of acetylsalicylic acid (100 mg) was administered intravenously. The
patient was treated with an intravenous infusion of sodium heparin in the first
24 hours (15 UI/kg/h), then he assumed acetylsalicylic acid (5 mg/kg) per os.
The procedure lasted 300 minutes. Fluoroscopic time was 203 minutes. Absorbed
dose and total dose area product were 1076 mGy and 6385 cGy/cm2, respectively.
The patient was discharged 72 hours after the procedure, in good clinical
condition. The jugular vein distension disappeared and pulse-oximetry
saturation increased until 88% (FiO2: 21%). Post-operative echocardiogram
showed an adequate blood flow into both the right and the left superior vena
cava and into the stent placed in the left pulmonary artery. Stenosis of pulmonary arteries
occurs in 2–3% of patients with congenital heart disease [1] and it can be
discrete or associated with long hypoplasic arterial tracts. The development of
scars at the site of trans-pulmonary patches or at the anastomotic sites on the
pulmonary arteries (in the cases of systemic-to-pulmonary shunts or Glenn
shunts) is the most common causes of post-surgical pulmonary arterial stenosis.
Complex surgical interventions (as an arterial switch, Norwood operation,
Damus–Kaye–Stansel procedure) may determine stretch or distortion of pulmonary
branches by developing a hemodynamically significant stenosis. A more
aggressive approach is necessary to pulmonary arterial stenosis in patients who
are undergoing to Fontan operation [7], since an adequate function of Fontan
circuit requires low pressure in the pulmonary circulation and a balanced blood
flow through the pulmonary arteries [8]. In this case, the severe stenosis
of left pulmonary artery would have compromised a right function of Fontan
circulation since the venous blood flow draining from the inferior vena cava
would be distributed to right pulmonary artery predominantly. It was needed to
stent the hypoplasic vessel to achieve a balanced pulmonary circulation. The
bad choice of stent length has compromised and complicated the procedure.
Probably the turbulent flow in the site of left-side superior vena cava
anastomosis favoured the migration of stent from the balloon despite a large
delivery system used (Mullins sheath 8 Fr, while the premounted vascular stent
required a 6 Fr sheath). Kakisis et al. describe a case of a stent dislodged
from the original position (in the left brachiocephalic vein) into the left
lower lobe pulmonary artery, where he adopted a successful strategy of “wait
and see” (the patient was anticoagulated and the stent left “in situ”). In our
case, it was impossible to adopt the same strategy because the unexpanded and
dislodged stent did not make possible the enlargement of the stenotic left
pulmonary artery. The choice to retrieve the stent percutaneously was
complicated by the severe stenosis and by the inability of the anchored stent
to cross over the narrowest segment. The presence of the left-side superior
vena cava and its anastomosis site beyond the narrowest tract allowed the
retrieval of loss stent. The procedure
was completed by releasing an adequate stent into the left pulmonary artery
with good hemodynamic and angiographic result.
In literature, several cases
about the retrieval of stents migrated into a pulmonary artery are described,
but all these ones concern adult patients, and the stent dislodged from a
central o peripheral vein to the pulmonary artery [9-11]. Furthermore, in these
cases, the pulmonary arteries showed a good diameter without stenotic or
hypoplasic segment. The only case about the retrieval of a stent in an infant
is described by Kobayashi et al. and it is about a transcatheter retrieval of a
stent embolized into the right ventricle [12]. In our experience, this is the
first case described about a stent migration into the left lower lobe pulmonary
artery in a child with a complex congenital heart disease (an univentricular
heart physiology), where the dislodgement of stent beyond the narrowest tract
of vessel and complex anatomy of patients complicated the strategies of
retrieval.
The angioplasty and stenting of
pulmonary arteries is a diffuse percutaneous procedure although not free from
complications, above all in patients with complex congenital heart disease
where the different anatomy, the previous surgical operations and the sites of
turbulent flow can compromise the results of procedure. The knowledge and the choice
of right materials and strategies are necessary to avoid and to solve dangerous
complications that can arise during the interventional catheterization.
1. Trivedi
KR and Benson LN. Interventional strategies in the management of peripheral pulmonary
artery stenosis (2003) J Intervent Cardiol 16: 171-188. https://doi.org/10.1046/j.1540-8183.2003.08031.x 2. Van
Gameren M, Witsenburg M, Takkenberg JJM, Boshoff D, Mertens L, et al. Early
complications of stenting in patients with congenital heart disease: a
multicentre study (2006) Eur Heart J 27: 2709-2715. https://doi.org/10.1093/eurheartj/ehl328 3. Brilakis
ES, Best PJM, Elesber AA, Barsness GW, Lennon RJ, et al. Incidence, retrieval
methods, and outcomes of stent loss during percutaneous coronary intervention:
a large single-center experience (2005) Cathet Cardiovasc Intervent 65: 333-340.
https://doi.org/10.1002/ccd.20449 4. Alomar
ME, Michael TT, Patel VG, Altomare CG, Rangan BV et al. Stent loss and
retrieval during percutaneous coronary interventions: a systematic review and
meta-analysis (2013) J Invasive Cardiol 25: 637-641. http://dx.doi.org/10.1016/S0735-1097(13)61649-6 5. Eggebrecht
H, Haude M, Von Birgelen C, Oldenburg O, Baumgart D, et al. Nonsurgical
Retrieval of Embolized Coronary Stents (2000) Cathet Cardiovasc Intervent 51: 432-440.
https://doi.org/10.1002/1522-726X(200012)51:4%3C432::AID-CCD12%3E3.0.CO;2-1 6. Ashwath
R, Gruenstein D and Siwik E. Percutaneous stent placement in children weighing
less than 10 kilograms (2008) Pediatr Cardiol
29: 562-567. https://doi.org/10.1007/s00246-007-9141-8 7. Franco
E, Domingo EJB, Del Val VA, Silva LGG, Del Cerro Marín MJ, et al. Percutaneous
interventions in Fontan circulation (2015) Int J Cardiol Heart Vasc 8: 138-146.
https://dx.doi.org/10.1016%2Fj.ijcha.2015.06.008 8. Gewillig
M. The Fontan Circulation (2005) Heart 91: 839-846. https://dx.doi.org/10.1136%2Fhrt.2004.051789 9. Kakisis
JD, Vassilas K, Antonopoulos C, Sfyroeras G, Moulakakis K, et al. Wandering
stent within the pulmonary circulation (1932) Ann Vasc Surg 28: 9-12. https://doi.org/10.1016/j.avsg.2014.06.074 10. Balasubramaniyam
N, Garg J, Rawat N, Chugh S, Mittal V, et al. Dual stent migration to the heart
and pulmonary artery (2014) Am J Ther 21: 199-203. https://doi.org/10.1097/MJT.0b013e3182785fc3 11. Dashkoff
N, Blessios GA and Cox MR. Migration of covered stents from hemodialysis A-V
access to the pulmonary artery: percutaneous stent retrieval and procedural
trends (2010) Catheter Cardiovasc Interv 76: 595-601. https://doi.org/10.1002/ccd.22553 12. Kobayashi
D, Singh HR, Turner DR, Forbes TJ and Gowda ST. Transcatheter retrieval and
repositioning of embolized stent from the right ventricle in an infant (2012)
Tex Heart Inst J 39: 639-643. Mario Giordano, Pediatric Cardiology, University of Campania, Italy, Tel:+39-0815373090, E-mail: giordanomario1123@gmail.com Giordano M, Gaio G, Bigazzi MC and Russo MG.Pulmonary arterial stenting complication: a case report about the retrieval of a stent lost in a child with bilateral glenn shunt (2018) Clinical Cardiol Cardiovascular Med 2: 1-4. Stent loss, Pulmonary artery stenting,
Cavopulmonary Glenn shunt, Complications.Pulmonary Arterial Stenting Complication: A Case Report about the Retrieval of a Stent Lost in a Child with Bilateral Glenn Shunt
Abstract
Full-Text
Introduction
Case report
Discussion
Conclusion
References
*Corresponding author:
Citation:
Keywords