Case Report :
Hannah Jethwa, Maaz Rana,
Jamie Kitt, Sarah Menzies, Alan Steuer and Simona
Gindea Systemic
Lupus Erythematosus (SLE) is an autoimmune disease characterized by multi-organ
damage mediated by immune complexes and autoantibodies. It often presents with
patients being non-specifically unwell and there is a wide range of
differential diagnoses, one of which includes chronic infections such as Tuberculosis
(TB). Exudative pericarditis is a relatively common feature in SLE and is
present in up to 62% of patients on autopsy, although only a quarter of
patients are symptomatic; constrictive pericarditis is a very rare feature of
SLE. We report a 49 year old gentleman who was diagnosed with SLE which
presented with features of constrictive pericarditis following an extensive
period of investigations for TB. We
report a 49 year old man who, other than having an unprovoked pulmonary
embolism in 2012, is otherwise fit and well. He is originally from Gambia but
has been working as a chef in the United Kingdom for the past 25 years. He last
travelled to Gambia in 2014, where he spent two weeks. He does not drink
alcohol and has never smoked. He has a family history of sickle cell disease,
but nil else of note. He presented to hospital in December 2015 with a several
week history of malaise, lethargy and shortness of breath. This was associated
with a cough which was occasionally productive of clear sputum, and over this
period he had a reduced appetite and had lost half a stone in weight. On
examination he was cachectic and had bilateral cervical lymphadenopathy. He
reduced bi-basilar air entry on his chest and abdominal examination revealed
hepatomegaly. Cardiovascular examination was unremarkable, with no evidence of
a murmur or pericardial knock. He had no signs of clubbing or peripheral
oedema. No skin rashes were noted. Bedside observations were stable other than
tachycardia (112 Beats per Minute (bpm)); of note, blood pressure was normal. His
initial investigations showed chronic microcytic anaemia with a Mean
Corpuscular Volume (MCV) of 71 fl. He had raised inflammatory markers with C-Reactive
Protein (CRP) of 73mg/L and Erythrocyte Sedimentation Rate (ESR) of 15 mm/hr.
Sputum cultures including those for Acid-Fast Bacilli (AFB) were negative.
Chest X-ray demonstrated bilateral pleural effusions with bi-basal
consolidation. Further
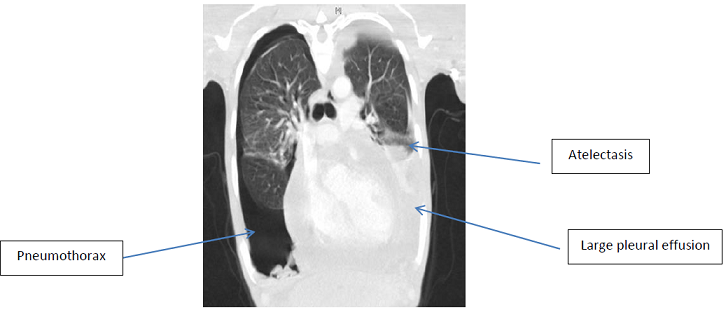
investigation including a Computer Tomography (CT) of his chest revealed
enlarged axillary, cervical and supraclavicular lymph nodes as well as
pericardial and bilateral pleural effusions with a right sided pneumothorax. He
then went on to have an echocardiogram which showed moderate circumferential
pericardial effusion with fibrinous strands; his ejection fraction was 64%.
There were no echocardiographic features of tamponade at that point, although
he had septal bounce and Tissue Doppler Imaging (TDI) patterns across the
mitral and tricuspid inflows were consistent with a constrictive effusive
picture. Intravenous Cholangiogram (IVC) at this time was <2 cm and
collapsed ~ 50%, which represents normal filling pressures. The main differential diagnosis at this stage was pulmonary
tuberculosis complicated by pleural and pericardial effusions and a pneumothorax.
Upon review by the respiratory team, he was started on the full
anti-tuberculosis treatment as well as 60mg of prednisolone (equivalent to 30mg
due to Rifampicin interactions) for the pericardial involvement. His left
pleural effusion was drained for symptomatic relief. The pleural fluid was
exudative and lymphocytic with a protein of 55.3 g/L, LDH of 178 u/L and few
White Blood Cells (WBC), 95% of which were lymphocytes. The fluid glucose was
6.3 mmol/L. An incision biopsy of his left axillary lymph node was performed,
which revealed reactive lymphadenitis. pleural fluid, serial sputum and lymph
node fluid cultures were, however, negative for TB (Figure 1). He
was discharged from hospital when his symptoms had improved with a plan to continue
TB quadruple therapy and a weaning regime of steroids (10 mg every 5 days). At
subsequent outpatient appointments in the TB clinic, he was noted to be making
good recovery. He started gaining weight and appetite returned to normal. His
pleural and pericardial effusions also improved and this reflected in
improvement in his cough and breathing. His cervical and supraclavicular nodes
had also shrunk in repeat imaging. Six weeks into his anti-TB treatment when he
was on 10mg prednisolone, his symptoms relapsed and he developed further
anorexia, weight loss and fatigue. At this stage he also experienced
significant shortness of breath on minimal exertion and orthopnea. He was
thereafter reviewed in the cardiology clinic as an outpatient and was noted to
be short of breath at rest (Respiratory Rate (RR)-24) and remained tachycardic
(120bpm) but normotensive with a positive Kussmauls sign, suggesting limited
right ventricular filling. An immediate trans-thoracic echocardiogram was
performed which showed a recurrence of his pericardial effusion (1.5 cm
globally) with semi-fibrinous material and progression of his constrictive
physiology. As
well as the septal bounce and TDI patterns seen on the first echocardiogram,
there was now a dilated inferior vena at 3.3 cm cava with flow reversal into
the hepatic veins on color doppler imaging. The Left Ventricle (LV) remained
non-dilated and Ejection Fraction (EF) was preserved. The constrictive picture
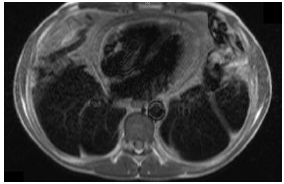
was later confirmed with cardiac Magnetic Resonance Imaging (cMRI) which
demonstrated circumferential thickening of the pericardium (13 mm) with
features of inflammation and constriction associated with a small pericardial
effusion and normal myocardium. The inferior vena cava and hepatic veins were
severely dilated. The left and right ventricles were noted to be small and he
had an LV EF of 70% on cMRI (Figure 2). Due
to a clinical relapse despite an extensive course of anti-TB treatment,
diagnosis was reconsidered with further differentials such as chronic infection
(toxoplasma, fungal, parasitic), haematological malignancy and systemic
autoimmune diseases and investigations performed accordingly. The prednisolone
dose was increased back to 50mg daily. TB treatment continued as diagnostic
uncertainty at that time, and the concern that if TB were a co-factor then
flare of this disease with immunosuppression may be potentially serious. Toxoplasma,
schistosoma and mycoplasma serology was negative and haematological malignancy
was unlikely based on the prior lymph node biopsy result. Malignancy markers
(AFP, CA125, CA19, CEA, HCG and PSA) were negative, and anti-IgG4 level was
normal. The autoimmune screen, however, revealed a positive Anti-Nuclear
Antibody (ANA) of 1:640 with a fine speckled pattern, positive anti-dsDNA with
a titer of 30, positive anti-Smith antibody and normal C3 and C4 levels. His
urine analysis showed trace of blood, and significantly raised urine protein:
creatinine ratio at 177 mg/mmol. In keeping with the American College of
Rheumatology (ACR) diagnostic criteria, Systemic Lupus Erythematosus (SLE) was
then diagnosed based on the presence of pericarditis, proteinuria, positive ANA
and anti-dsDNA Ab. The prednisolone dose was increased to 60 mg (30 mg
equivalent due to Rifampicin) and hydroxychloroquine was initiated. Upon
discussion with Cardiology, 0.5 mg colchicine twice daily was also started for
symptomatic relief of constrictive pericarditis. After months of treatment, his
fatigue and systemic symptoms had improved, but he remained symptomatic from
constrictive effusive pericarditis, with ongoing symptoms and signs of right
sided heart failure. A
subsequent uncomplicated pericardiectomy was performed, which resulted in a
complete resolution of the signs of right sided heat failure and shortness of
breath. Pericardial biopsy results showed granulomatous inflammation and
fibrosis; TB cultures were negative. A subsequent renal biopsy showed features
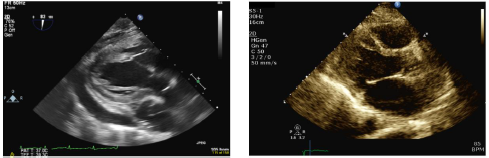
of membranous lupus nephritis. A repeat echocardiogram following the procedure
showed mild septal bounce with no effusion but preserved biventricular function
and normal sized ventricles, consistent with a significant resolution of his
constrictive physiology post pericardiectomy (Figure 3). To
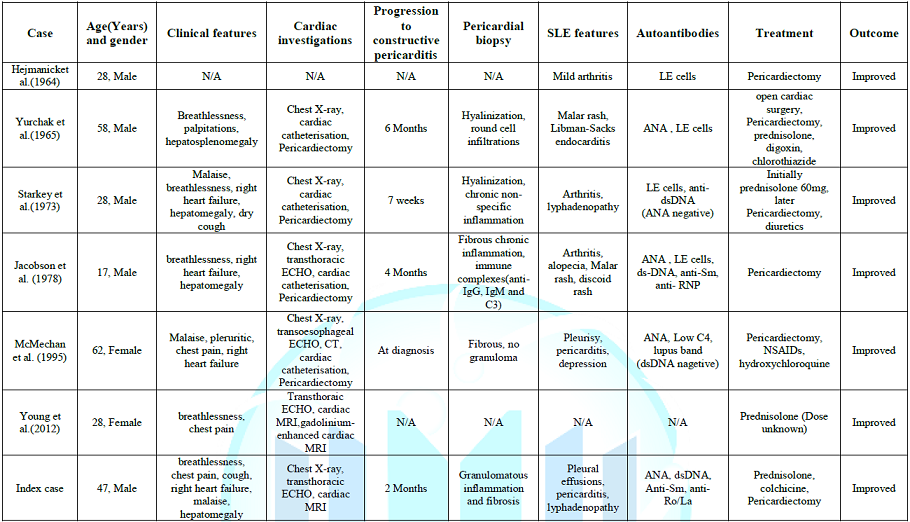
the best of our knowledge, only six cases of systemic lupus erythematosus with
constrictive pericarditis have been reported in English literature. Of these,
four patients were male and two female, with a median age of 38 years (range
17-58 years). Only two patients had a prior diagnosis of SLE and TB was part of
the differential diagnosis in at least five cases. The progression from
exudative pericarditis to constrictive pericarditis ranged from one week to six
months and some authors postulated that it might have been triggered by rapid
reduction of prednisolone dose. Only one of these six cases had an
effusive-constrictive pericarditis similar to our case. The diagnosis of
constrictive pericarditis was confirmed by echocardiography, right ventricular
catheterization or cardiac MRI (Table 1)
[1-7]. Five
of six patients underwent pericardiectomy and pericardial biopsy reports were
described in four cases. Overall, the histopathological findings were
non-specific: two biopsies showed hyalinization and either round-cell
infiltration or non-specific inflammation, other two demonstrated fibrosis with
inflammation but no granulomas [2,4-6]. Immunohistochemistry was performed in
the case described by Jacobson et al. [2] and it revealed the presence of
anti-IgG, IgM and C3 in pericardial tissue. Our patient is the only case that
demonstrated granulomatous inflammation and fibrosis; still the TB cultures
were negative. Cardiac symptoms resolved with steroid treatment alone in only
one case; in five patients, as well as in our patient, pericardiectomy was
performed leading to symptoms resolution [3]. All patients survived. Although
the most common causes of constrictive pericarditis are infectious or
idiopathic, this case highlights that underlying connective tissue diseases
should also be considered as a differential diagnosis, especially in those
cases with no proven etiology and without improvement with antimicrobial or
antiviral treatment. Although constrictive pericarditis is a rare feature in
SLE, a high index of suspicion is needed in patients who have worsening
shortness of breath despite a normal initial echocardiogram. In such cases, a
Cardiology opinion and cardiac MRI may be of value in identifying underlying
complications. Although it is difficult to make conclusions regarding gender
prevalence when only seven cases have been reported we note that five (71%) of
these patients were male; it is well-known that SLE has a female preponderance
though male patients with SLE often have more severe disease, which may account
for the higher proportion of male patients with constrictive pericarditis in
our literature review. In such patients with constrictive pericarditis, even
though corticosteroid treatment may partially alleviate the symptoms a Pericardiectomy
is likely to be required for definitive treatment. While a pericardial biopsy
might reveal non-specific features of acute or chronic inflammation, it should
be performed in all cases if possible, in order to exclude underlying
infection. 2. Jacobson
EJ and Reza MJ. Constrictive pericarditis in systemic lupus erythematosus.
Demonstration of immunoglobulins in the pericardium (1978) Arthritis Rheum 21: 972-974.
https://doi.org/10.1002/art.1780210815 3. Young
Oh J, Chang SA, Choe HY and Kim DK. Transient constrictive pericarditis in
systemic lupus erythematosus (2012) European Heart J Cardiovascular Imaging 13:
793. https://doi.org/10.1093/ehjci/jes052 4. Yurchak
PM, Levine SA and Gorlin R. Constrictive pericarditis complicating disseminated
lupus erythematosus (1965) Circulation 31: 113-118. https://doi.org/10.1161/01.CIR.31.1.113 5. Starkey
RH and Hahn BH. Rapid development of constrictive pericarditis in a patient
with systemic lupus erythematosus (1973) Chest 63:448-450. https://doi.org/10.1378/chest.63.3.448 6.
Mcmechan
SR, McClements BM, McKeown PP, Webb SW and Adgey AA. Systemic lupus
erythematosus presenting as effuso-constrictive pericarditis (1995)
Postgraduate Medical J 71: 627-629. https://doi.org/10.1136/pgmj.71.840.627 7. Hejmancik
MR, Wright JC and Quint R. The cardiovascular manifestations of systemic lupus
erythematosus (1964) American Heart J 68: 119-130. https://doi.org/10.1016/0002-8703(64)90248-0 Hannah Jethwa, Department of Rheumatology,
Addenbrookes Hospital, Cambridge, United Kingdom, Tel: +01223 217457 E-mail: hannahjethwa@nhs.net Jethwa H, Rana M,
Kitt J, Menzies S, Steuer A, et al. Constrictive pericarditis as the first
presentation of systemic lupus erythematosus (2019) Rheumatic Dis Treatment J 1:
10-12. Lupus, Autoimmune disease, Bilateral cervical lymphadenopathy,Cardiovascular examination.Constrictive Pericarditis as the First Presentation of Systemic Lupus Erythematosus
Abstract
Full-Text
Case Summary
Discussion
Conclusion
References
*Corresponding author
Citation
Keywords