Research Article :
In this paper
Thermodynamic calculation is shown. We have found simulation for phase diagram,
Gibbs free energy and Activity curve at different temperatures (1200 K, 1225 K
and 1250 K). Phase diagrams, Gibbs free-energy and the component activities of
(Fe-Co) alloys system were calculated by Calphad method. Results show that the
values of Gibbs energy were negative, which shows the stability of (Fe-Co).
Negative deviation had occurred from Raoults Law in activities, which indicates
that there is strong interaction between Fe and Co in (Fe-Co) alloy. By
increasing the temperature the activity increases and deviation in activity
decreases. For all the thermodynamic calculations the Thermo-Calc software,
databases and Calphad method have used. The study on Experimental
measurement of thermodynamic properties and phase equilibrium for some systems
is very difficult since these systems may be costly. For these systems using
modeling and simulation is of great interest. This modeling and simulation
reduces the time and to find equilibrium conditions for binary and
multi-component systems. J.W. Gibbs was established the relationship between
thermodynamics and phase equilibria [1]. Iron-cobalt alloy has magnetic
properties, with high Curie temperatures, the highest saturation magnetizations
and high permeability etc. Elmen discovered these alloys in 1929 [2]. Also (Fe-Co) alloy has been
studied by Ellis and Greiner [3]. Normanton et al [4]. In 2002 by Ohnuma et al
[5]. This alloy has applications in electric engine, which contain magnetic
materials [6]. And it is now a good thermodynamic explanation of it. Thermo-Calc is powerful and
flexible software to performing various kinds of thermodynamic and phase
diagram calculations. This software can helpful to solve difficult problems
interaction of many elements and phases that shows highly non ideal behavior
[7]. The first version of thermo-calc
was shown in 1981. In 2002 modern version of thermo- calc was present. The
first description on thermo-calc was in 1985 [8]. Calphad stands for calculation of
phase diagram. The Calphad method is used to find the thermodynamic properties
of different materials system. The CALPHAD method was established as tool for
phase equilibria of different multicomponent systems and for treating
thermodynamics. For the calculations, the modules reported are used [9]. CALPHAD
method has been employed for the material properties [10]. Recently the Calphad
approach has been applied to a systems having more phase-based properties, as
reported molar volume, elastic moduli and the database for processing these
functions have been developed [11]. For materials the Calphad method can be
used to describing the composition, temperature of optical activity,
thermo-electric and acoustic properties [12]. All the calculations have been
done by Calphad method. A thermodynamic calculation in
Fe-Co has been done using Thermo-Calc Software and Databases. The results of
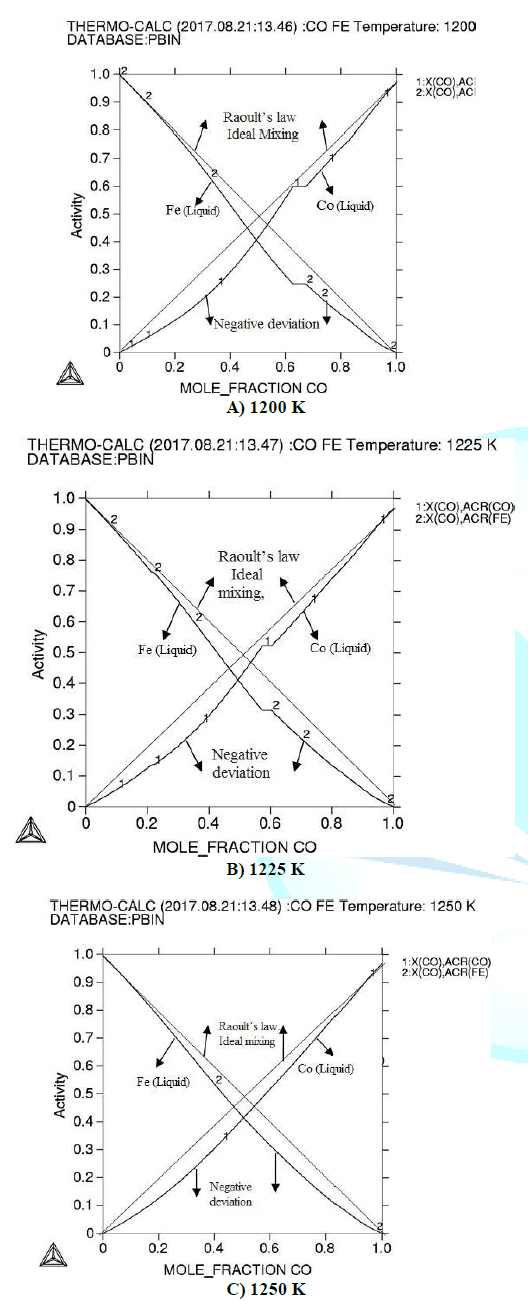
calculated activities in (Fe-Co) at 1200 K, 1225 K and 1250 K are presented in
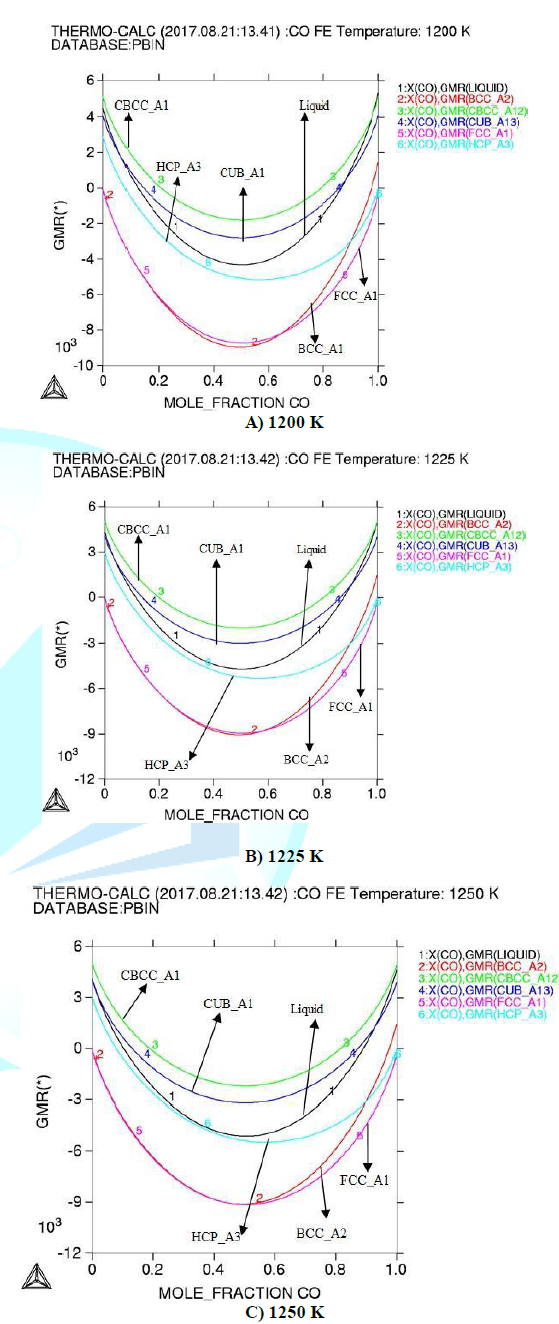
fig1. Gibbs energy for the investigated binary system is shown in figure2. In
this paper the reaction, equilibria, phase diagram and figures modules were
used. In liquid (Fe-Co) alloy there
negative deviation can occur in activities. The activities increase
proportionally with increasing of the temperature and deviation decreases in
activity according to the Raoults law [13]. Activities show that there is
strong interaction between the atoms of the alloy. Considering calculated
integral thermodynamic properties both the alloys show negative values for
Gibbs energy which represents the stability of (Fe-Co) alloy according to [14].
Gibbs energy decreases with increasing the temperature. Figure 1: Activity of Fe-Co alloy at different temperatures
(a) 1200 K (b) 1225 K (c) 1250 K. Table1: Various Thermodynamic properties of (Fe-Co) alloy at
1200 K, 1225 K and 1250 K. Phase diagrams of the binary
systems obtained by thermo-calc software and databases are shown in Fig3. In
phase diagrams different phases can occur at different temperatures and at
various concentrations. Comparison with referent data shows good accordance with
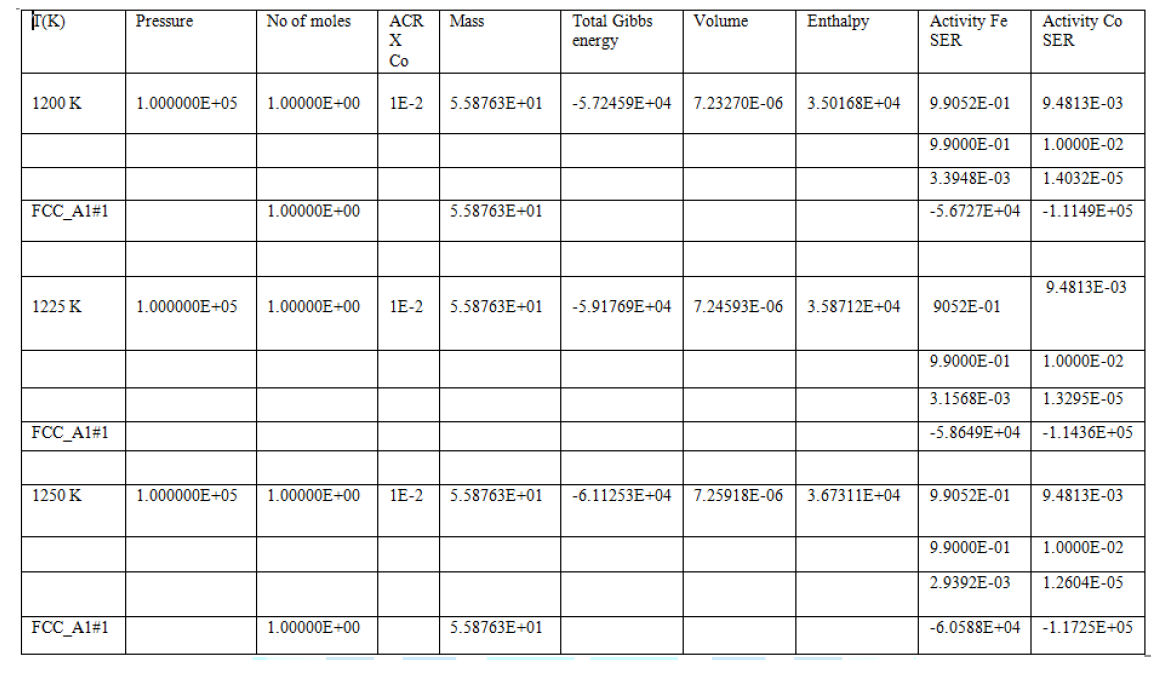
available phase diagrams in literature. Figure 3: Phase diagram of Fe-Co alloy. In the above Table 1 pressure and mass are constant. However other thermodynamic
properties i.e. Gibbs free energy, activity, enthalpy and volume are change at
different temperatures. In table it is clear that increasing the temperatures
then activity increases and total Gibbs energy decreases, which shows
proportionality between temperature and thermodynamic parameters. The highest
negative value of Gibbs energy is observed at 1250 K showing stability of
(Fe-Co) binary alloys system; the enthalpy proportionally increases with
temperature due to the fact that the heat content of the system increases with
temperature. At the three temperatures (1200 K, 1225 K and 1250 K) there
(FCC_Al#l) phase can occurs, which is the austenite phase shows solid solution
of the alloy (Fe-Co). At 1250 K we observed maximum enthalpy, which shows the
capability of system at high temperature and heat provide high stability of
alloys. A thermodynamic property of
Ferric base alloy system (Fe-Co) alloy has been found by Thermo-Calc software.
It is indicated that thermo-calc software play important role in the design of
structure and functional of Fe base alloy. The thermodynamic calculations in
binary alloy (Fe- Co) at 1200 K, 1225 K and 1250 K were determined. The binary
system has negative values for Gibbs energy. The total Gibbs energy decreases
with increasing temperature. At 1250 K, highest negative value of Gibbs energy
in (Fe-Co) alloy system is observed which shows that the system is more stable,
which increasing the level of stability, hardness, wear resistance, corrosion
resistance and other doping characteristics of alloy. Activity values of the
components are less than unity and show negative deviation from the Raoults
law. Increase the temperature then activity increases and deviation in activity
decreases, which indicates that there is strong interaction between the atoms
of (Fe-Co). At 1250 K it has better accordance with Raoults law in Fe-Co binary
system, which is important for engineering technology and metallurgy. A Phase diagrams for the
investigated binary system is calculated by the use of Thermo- Calc software
and database. Obtained results presented one good base for the further
thermodynamic analysis and developing in this group of innovative Fe base
alloys. The obtained and shown results for the (Fe-Co) system provide complete
thermodynamic optimization of this system. 1.
J Hertz. Josiah Willard Gibbs and
Teaching Thermodynamics of Material (History) (1992) J Phase Equilibria 13:
450-458. https://doi.org/10.1007/BF02665759 2.
GW Elmen. Magnetic material and
appliance (1929) US patent No. 1: 739-752. 3.
Ellis WC and Greiner ES.
Equilibrium relations in the solid state of their on-cobalt system (1941) Trans
AS 415-433. 4.
Normanton AS, Bloomfield PE, Sale
FR and Argent BB. A calorimetric study of iron-cobalt alloys (1975) Metal Sci
9: 510-517. http://dx.doi.org/10.1179/030634575790444658 5.
Ohnuma I, Enoki H, Ikeda O,
Kainuma R, Ohtani H, et al. Phase equilibria in the Fe-Co binary system (2002)
Acta Mater 50: 379-393. https://doi.org/10.1016/S1359-6454(01)00337-8 6.
Fingers RT and Rubertus CS.
Application of high temperature magnetic materials (2000) IEEE Trans Magnetics
36: 3373-3375. https://doi.org/10.1109/20.908805 7.
Andersson JO, Helander T, Hoglund
L, Shi P and Sundman B. Thermo-Calc & DICTRA, computational tools for
materials science (2002) Calphad 26: 273. https://doi.org/10.1016/S0364-5916(02)00037-8 8.
Sundman B, Jansson B and
Andersson JO. The Thermo-Calc databank system (1985) CALPHAD 9: 153-190. https://doi.org/10.1016/0364-5916(85)90021-5 9.
Xiong W, Hedstrom P, Selleby M,
Odqvist J and Thuvander M. An Improved Thermodynamic Modeling of the Fe-Cr
System Down to Zero Kelvin Coupled with Key Experiments (2011) Calphad 35: 355.
http://dx.doi.org/10.1016/j.calphad.2011.05.002 10. Saunders
N, Kucherenko S, Li X, Miodownik AP and Schille J. A new computer program for
Predicting materials properties (2001) J Phase Equilibria 22: 463-469. https://doi.org/10.1361/105497101770333036 11. Hallstedt
B, Dupin N, Hillert M, Hoglund L, Lukas H, et al. Thermodynamic models for
crystalline phases. Composition dependent models for volume, bulk modulus and
Thermal expansion (2007) CALPHAD 31:
28-37. https://doi.org/10.1016/j.calphad.2006.02.008 12. Gheribi
A and Charrand P. Application of the CALPHAD method to predict the thermal
conductivity in dielectric and semiconductor crystals (2012) CALPHAD 39: 70-79.
https://doi.org/10.1016/j.calphad.2012.06.002 13. Kostov
A, Friedrich B and Zivkovic D. THERMODYNAMIC CALCULATIONS IN ALLOYS Ti-Al,
Ti-Fe, Al-Fe and Ti-Al-Fe (2008) J Mining and Metallurgy 44: 49-61.
DOI:10.2298/JMMB0801049K 14. Waseem
Ullah Shah, Syed Mehmood Shah, Matiullah Khan, Dil Faraz Khan, Athanasios G.
Mamalis, et al. Thermodynamic Study Of Binary Alloy System (Co-Cr) Using
Calphad Method (2018) Surface Review and Letters. 25: 1850049. https://doi.org/10.1142/S0218625X1850049X CALPHAD; (Fe-Co) binary alloys; Thermo-Calc
software; Phase diagram; Activity curve; Gibbs curve, Thermodynamic
calculation.Thermodynamic Analysis and Calculations of (Fe-Co) Alloy by Modeling and Simulation using Thermo--Calc Software
Abstract
Full-Text
Introduction
Calphad
method
Results
and Discussion

Conclusion
References
Keywords