Research Article :
Mahendra Kumar Trivedi, Alice Branton, Dahryn Trivedi, Gopal Nayak, Ragini Singh, Snehasis Jana*
Toluic
acid isomers are widely used as a chemical intermediate in manufacturing of
dyes, pharmaceuticals, polymer stabilizers, insect repellent and other organic
synthesis. The aim of present study was to evaluate the impact of biofield
treatment on physical, thermal and spectroscopic properties of ortho isomer of
toluic acid (OTA). The OTA sample was divided into two groups, served as
control and treated. The treated group received Mr. Trivedi’s biofield
treatment. Subsequently, the control and treated samples were evaluated using
X-ray diffraction (XRD), differential scanning calorimetry (DSC),
thermogravimetric analysis/ derivative thermogravimetry (TGA/DTG), Fourier
transform infrared (FT-IR) and ultraviolet-visible (UV-Vis) spectroscopy. XRD
result showed 26.66% decrease in crystallite size in treated OTA sample as
compared to control. Furthermore, DSC analysis result showed that latent heat
of fusion was considerably reduced by 6.68% in treated OTA sample as compared
to control. However, an increase in melting point was observed in treated
sample. The melting point of treated OTA sample was found to be 107.96°C as
compared to control (105.47°C) sample. Moreover, TGA/ DTG studies showed that Tmax (temperature, at which
sample lost its maximum weight) was decreased by 1.21% in treated OTA sample as
compared to control. It indicates that vaporisation of treated OTA sample might
increase as compared to control. The FT-IR and UV-Vis spectra did not show any
significant changes in spectral properties of treated OTA sample as compared to
control. These findings suggest that biofield treatment has significantly
altered the physical and thermal properties of OTA, which could make it more
useful as chemical intermediate. Toluic acids are closest
homologues to benzoic acid. It is basically available in three isomeric forms i.e. ortho, meta, and para isomers. All
three isomers are colourless and crystalline substances, which are virtually
insoluble in water but soluble in organic solvents such as ethyl alcohol,
diethyl ether etc. Toluic acids can be obtained from toluidines and oxidation
of xylenes [1]. It is used as a raw material in the production of various
pharmaceutical drugs as well as an additive to the nutrient medium which is
used in cultivation of mold for production of benzylpenicillin [2].
The ortho isomer of toluic acid i.e. o-toluic acid (OTA) is used as an
intermediate for polymer stabilizers and pesticides. It is also used in
production of animal feed supplements and other organic chemicals like
pharmaceuticals, pigments and dyestuffs. It is used as raw material in
production of o-toluoyl chloride, o-tolunitrile etc. It is also used as
intermediate in production of 5-iodo-2-methylbenzoic acid [3].
OTA is used as intermediate in various chemical
reactions, where its rate of reaction plays a crucial role. Carballo et al. reported that rate of reaction in
organic compounds can be controlled by modulating the crystallite size [4].
Since, OTA is generally used as chemical intermediate in various reactions,
some alteration in its crystallite size and thermal stability may affect the
reaction kinetics and ultimately the percentage yield of end product [5]. After
considering the properties and applications of OTA, authors wanted to
investigate an economically safe approach that could be beneficial in order to
modify its physical and thermal properties.
Biofield
is the name given to the electromagnetic field that permeates and surrounds
living organisms [6]. It is scientifically termed as the biologically
produced electromagnetic and subtle energy field that provides regulatory and
communication functions within the human organism. Biomagnetic fields present
around human body can be measured through various techniques such as
electromyography, electrocardiography and electroencephalogram [7]. Thus, a
human has the ability to harness the energy from environment or Universe and
can transmit into any living or non-living object(s). The objects always
receive the energy and responding into useful way that is called biofield
energy and the process is known as biofield treatment. The concept of human
bioenergy has its origin thousands of year back, till date many recent biofield
therapies are in practice for their possible therapeutic potentials such as
enhanced personal well-being, improved functional ability of arthritis patient,
decreased pain and anxiety [8-10]. Biofield therapies are very popular in
holistic medicine heath care systems and are included in the National Center
for Complementary and Alternative Medicine (NCCAM), which is part of the
National Institute of Health (NIH). NCCAM places biofield therapy (putative
energy fields) as a subcategory of energy medicine among complementary and
alternative medicines [11,12]. These healing treatments suggest their mechanism
upon modulating patient-environmental energy fields. Mr. Trivedis biofield
treatment (The Trivedi Effect®) is well
known and significantly studied in different fields such as microbiology,
agriculture, and biotechnology [13-19]. Recently, impact of biofield treatment
on atomic, crystalline and powder characteristics as well as spectroscopic
properties of different materials was studied and alteration in physical,
thermal and chemical properties was reported [20-22]. Hence, based on the
outstanding results obtained after biofield treatment on different materials
and considering the applications of OTA, the present study was undertaken to
evaluate the impact of biofield treatment on physical, thermal and
spectroscopic properties of OTA. o-Toluic acid (OTA) was procured from S D Fine ChemicalsPvt. Ltd., India. The sample was
divided into two parts; one was kept as a control, while other was subjected to
Mr. Trivedis biofield treatment and coded as treated sample. The treatment
group in sealed pack was handed over to Mr. Trivedi for biofield treatment
under standard laboratory conditions. Mr. Trivedi provided the treatment
through his energy transmission process to the treated group without touching
the sample. The biofield treated samples were returned in the similar sealed
condition for further characterization using XRD, DSC, TGA, FT-IR and UV-Vis
spectroscopic techniques.
G = kλ/(bCosθ) Here, λ is the wavelength of
radiation used, b is full width half maximum (FWHM) of peaks and k is the
equipment constant (=0.94). However, percent change in crystallite size was
calculated using the following equation:
Percent change in crystallite size = [(Gt-Gc)/Gc] ×100
Where, Gc and Gt are
crystallite size of control and treated powder samples respectively.
Thermogravimetric analysis/ Derivative Thermogravimetry (TGA/DTG) Thermal stability of control and treated samples of
OTA was analysed by using Mettler Toledo simultaneous Thermo-gravimetric
analyser (TGA/DTG). The samples were heated from room temperature to 400ºC with
a heating rate of 5ºC/ min under air atmosphere. From TGA curve, onset
temperature
Tonset (temperature at which sample start losing weight) and from DTG curve, Tmax (temperature at which sample
lost its maximum weight) was recorded. Percent change in temperature at which maximum
weight loss occur in sample was calculated using following equation:
Where, Tmax, control and Tmax, treated are temperature at which maximum weight loss occurs in control and treated
sample, respectively.
For determination of spectroscopic characters, the
treated sample was divided into two groups i.e.
T1 and T2. Both treated groups were analysed for their spectral characteristics
using FT-IR and UV-Vis spectroscopy as compared to control OTA sample.
FT-IR spectra were recorded on
Shimadzus Fourier transform infrared spectrometer (Japan) with frequency range
of 4000-500 cm-1. The FT-IR spectroscopic analysis of OTA (control, T1 and T2) were
carried out to evaluate the impact of biofield treatment at atomic and
molecular level like bond strength, stability, rigidity of structure etc.
The UV-Vis spectral analysis was measured using
Shimadzu UV-2400 PC series spectrophotometer over a wavelength range of 200-400
nm with 1 cm quartz cell and a slit width of 2.0 nm. This analysis was
performed to evaluate the effect of biofield treatment on structural property
of OTA sample. The UV-Vis spectroscopy give the preliminary information related
to skeleton of chemical structure and possible arrangement of functional groups
[23].
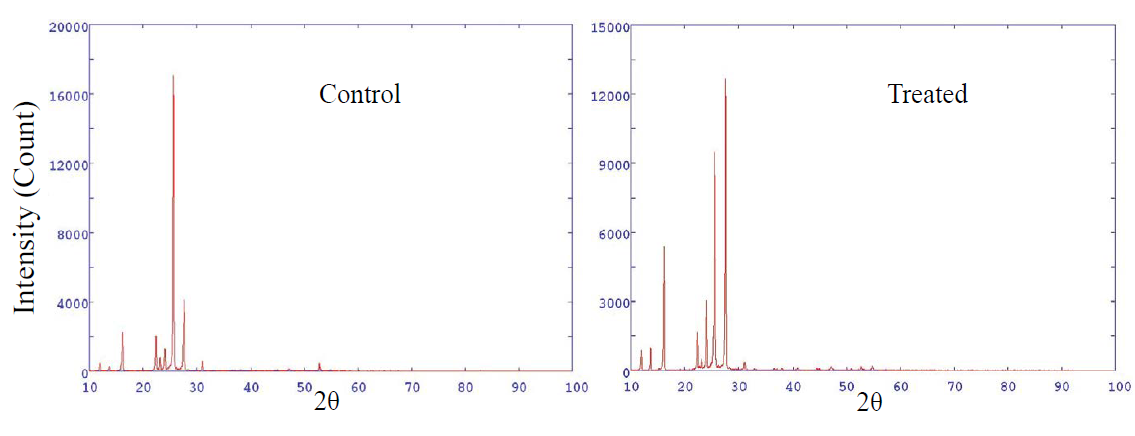
X-ray diffraction study was conducted to study the
crystalline nature of the control and treated samples of OTA. Figure A1 showed
the XRD diffractogram of control and treated samples of OTA. The control sample
showed intense crystalline peaks at 2θ equals to 16.20º, 22.45º, 24.14º,
25.57º, 25.70º and 27.68º. Intense peaks indicated the crystalline nature of
OTA. Whereas, the XRD diffractogram of treated OTA showed peaks with altered
intensity as compared to control sample. The XRD diffractogram of treated OTA
showed crystalline peaks at 2θ equals to 16.17º, 22.40º, 24.07º, 25.31º, 25.60º
and 27.61º (Figure 1). In addition, the crystallite size was found to be 93.03
and 68.23 nm in control and treated OTA, respectively (Figure 2). It indicates
that crystallite size was decreased by 26.66% in treated OTA as compared to
control.
It is presumed that biofield energy may be absorbed
by the treated OTA molecules which may lead to formation of more
symmetrical crystalline long range pattern; that caused increase in
intensity of some peaks. Also treated samples of OTA showed decreased
crystallite size as compared to control, which suggest that biofield energy
might induce strain in lattice and that possibly results into fracturing of
grains into sub grains and hence results decreased crystallite size. As OTA is
used as intermediate in synthesis of many pharmaceutical compounds, the
decrease in crystallite size may lead to fasten the rate kinetics which
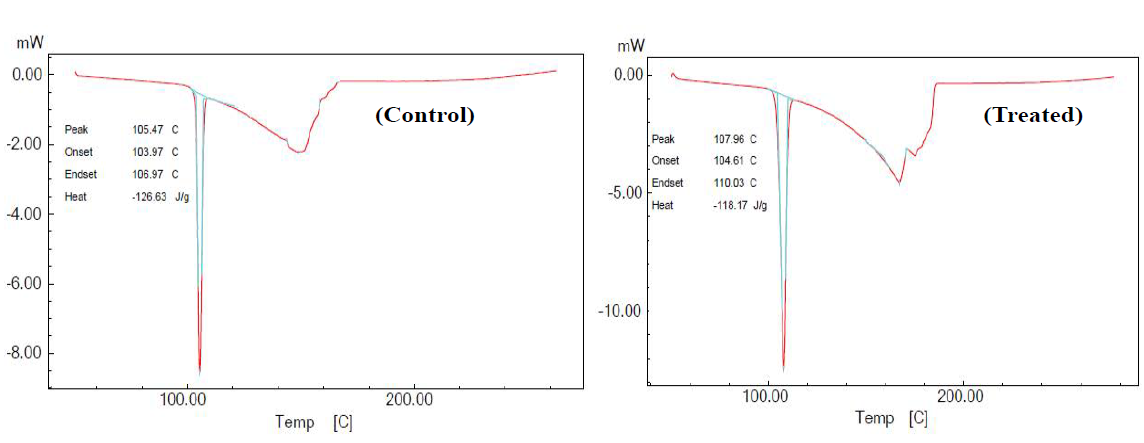
ultimately enhances the percentage yield of end products [5]. Differential scanning calorimetry (DSC) was used to
determine the latent heat of fusion and melting temperature in control and
treated sample of OTA. The DSC thermograms of control and treated samples of
OTA are shown in Figure 3. In a solid, substantial amount of interaction force exists
in atomic bonds to hold the atoms at their positions, thus a sufficient amount
of energy is required to change the phase from solid to liquid, known as latent
heat of fusion (ΔH). Further, the energy supplied during phase change i.e. ΔH is stored as potential energy of
atoms. However, melting point is related to kinetic energy of the atoms. Figure 1: XRD diffractogram of control and treated sample of
o-toluic acid. Figure 2: Crystallite size of control and treated sample of
o-toluic acid. Figure 3: DSC thermogram of control and treated sample of
o-toluic acid Figure 5: TGA thermogram of control and treated samples of
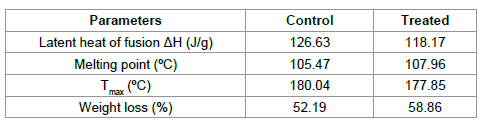
o-toluic acid. Data showed that ΔH was reduced from 126.63 J/g (control) to 118.17 J/g
in treated OTA. It indicates that ΔH was decreased by 6.68 % in treated sample
as compared to control. However, the melting point of treated OTA was increased
from 105.47°C (control) to 107.96°C. Thus, data suggest that melting point was
increased by 2.36% as compared to control (Figure 4). Previously, our group
reported that biofield treatment has altered the latent heat of fusion and
melting point in lead and tin powder [25]. The reduction in ΔH revealed that
treated OTA probably have extra internal energy in form of potential energy as
compared to control, which might be transferred through biofield treatment.
This potential energy might be stored in treated OTA molecules, which results
in lowering of ΔH in treated sample as compared to control. Besides, the
increase of melting point in treated OTA suggests that kinetic energy and
thermal vibrations of molecules probably altered after biofield treatment. In
addition, the sharpness of the endothermic
peaks showed good degree of crystallinity in control and treated samples of
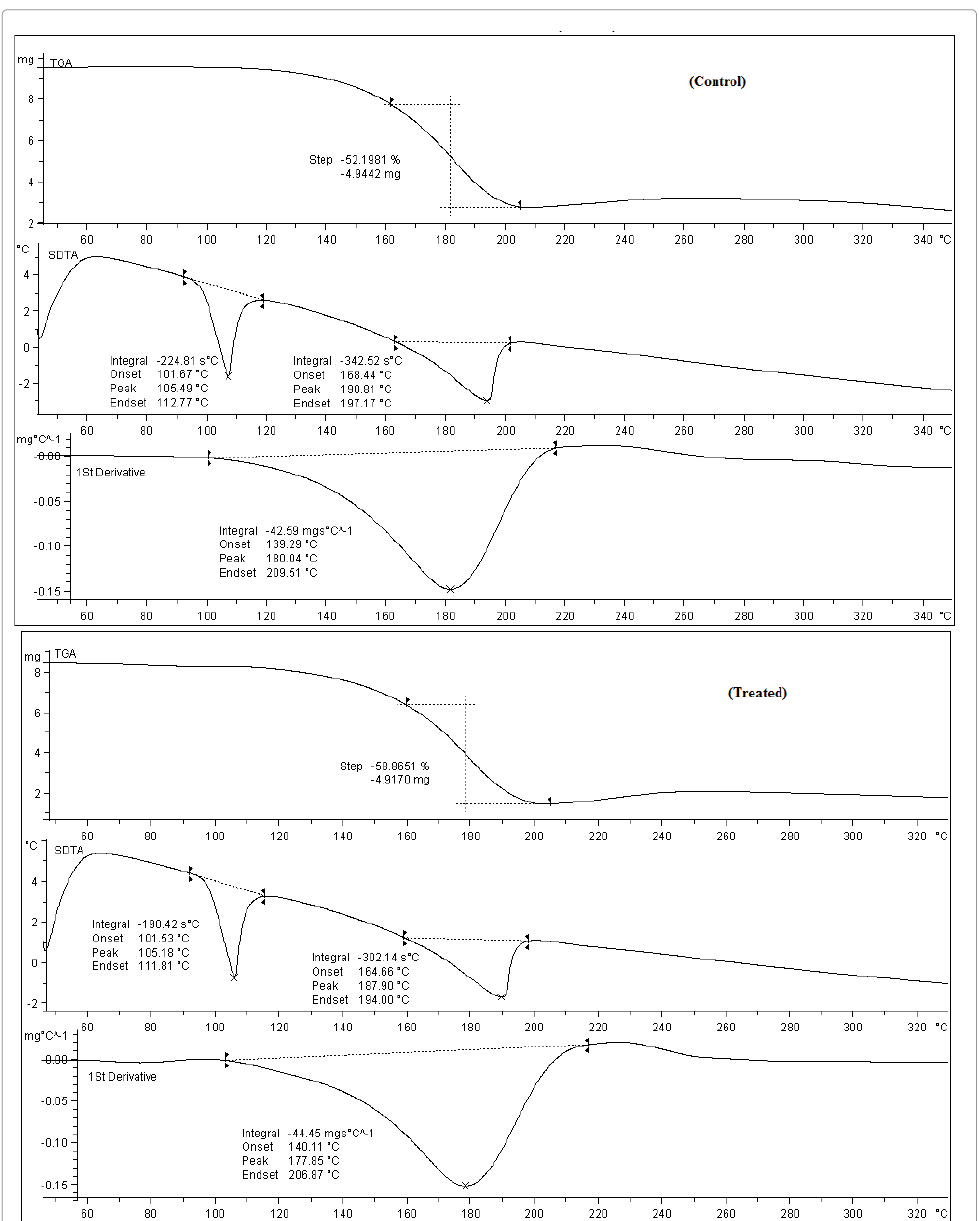
OTA. TGA/DTG thermogram of control and biofield treated
samples are summarized in Table 1. TGA thermogram of control OTA sample (Figure
5) showed that it started losing weight around 160°C (onset) and stopped at
207°C (end set). However, the treated OTA also started losing weight near to
160°C (onset) and terminated at 207°C (end set). It indicates that no significant
change was found in onset and endset temperature of treated OTA as compared to
control. Furthermore, in this process, control sample lost 52.19% and treated
OTA sample lost 58.86% of its weight, which could be due to vaporisation of
OTA. Besides, DTG thermogram data showed that Tmax was found at 180.04°C in control
whereas, it was decreased to 177.85°C in treated OTA (Table 1). It indicates
that Tmax was decreased by 1.21% in treated OTA as compared to control.
Furthermore, the reduction in Tmax in treated sample of OTA with respect to control sample may be
correlated with increase in vaporisation of treated sample of OTA after
biofield treatment. It was previously reported that vapour phase reaction can
be more advantageous as compared to liquid phase reaction in terms of reaction
time, generation of objectionable amounts of odour and undesired by-products
[26,27]. Hence, biofield treated OTA can be used in those reactions as decrease
in vaporisation temperature may enhance the reaction kinetics and yield of end
product.
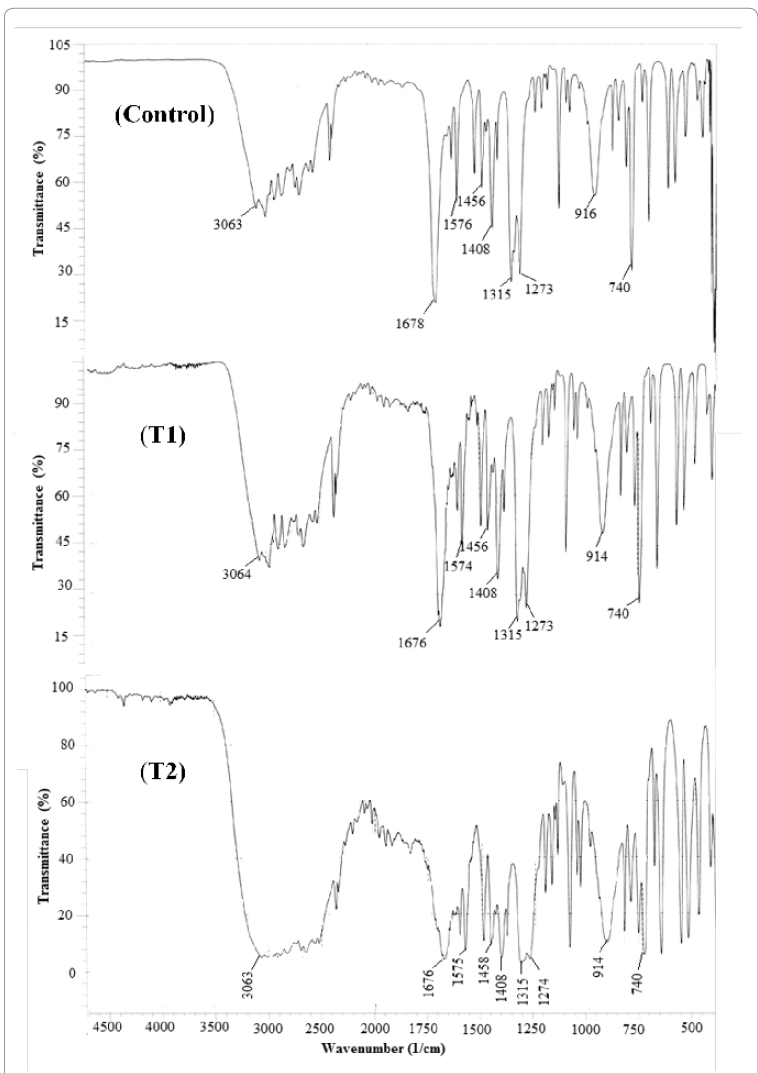
FT-IR spectra of control, T1 and T2 samples of OTA
are shown in Figure 6. The aromatic C-H stretching peak was appeared at 3063,
3064 and 3063 cm-1 in
control, T1 and T2 sample respectively. The C=O stretching (carboxylic acid)
peak appeared at 1678 cm-1 in
control and 1676 cm-1 in both
T1 and T2 samples. The peak due to aromatic ring stretching was appeared at
1576, 1574 and 1575 cm-1 in
control, T1 and T2 sample respectively. CH3 bending peak appeared at 1456 cm-1 in control and T1, and at 1458 cm-1 in T2 sample. C -C stretching peak was found at 1408 cm-1 in all three samples i.e. control, T1 and T2. Similarly C- O
stretching (carboxylic acid) peak appeared at 1315 cm-1 in all three samples i.e. control, T1 and T2. C -OH
stretching peak appeared at 1273 cm-1 in control and T1 sample and at 1274 cm-1 in T2 sample. O-H bending peak was found at 916 cm-1 in control and 914 cm-1 in both T1 and T2 sample. The
peak due to ortho substituted arene appeared at 740 cm-1 in all three samples (i.e. control, T1 and T2). The FT-IR
spectra were well supported by reference data [28].
Table 1: Thermal analysis of control and treated sample of
o-toluic acid Figure 6: FT-IR spectra of control and treated (T1 and T2)
samples o-toluic acid. The FT-IR spectroscopic study showed that no
alteration was found in terms of frequency of peaks of treated samples (T1 and
T2) however, intensity of peaks in T2 sample was slightly increased as compared
to control. It suggests that biofield treatment did not cause any alteration in
structural and bonding properties like bond strength, stability, rigidity of
structure etc.
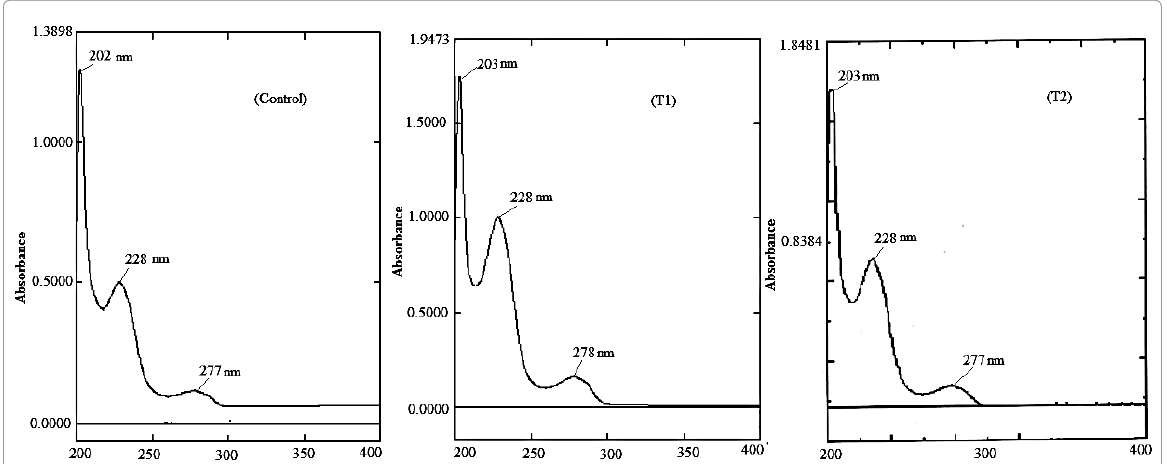
The UV spectra of control and treated samples (T1
and T2) of OTA are shown in Figure 7. The UV spectrum of control sample showed
three absorption peaks i.e. at 202,
228 and 277 nm and the spectrum was well supported by literature data [29]. The
UV spectrum of both treated samples of OTA (T1 and T2) also showed similar
absorption peaks as compared to control. In T1 sample, absorption peaks
appeared at 203, 228 and 278 nm whereas in T2 sample, peaks appeared at 203,
228 and 277 nm. It suggests that biofield treatment could not make any
alteration in chemical structure or arrangement of functional groups of treated
OTA samples.
Overall study showed influence of biofield
treatment on physical and thermal properties of OTA. XRD result showed that crystallite size was decreased by 26.66% in treated OTA sample as
compared to control, which might be due to fracturing of grains into sub grains
caused by lattice strain produced via
biofield energy. The reduction in crystallite size may lead to increase in
reaction kinetics of OTA which make it more useful as an intermediate compound.
Thermal analysis data revealed that latent heat of fusion was reduced by 6.68%
whereas, melting point was increased by 2.36% in treated OTA sample as compared
to control. TGA/DTG studies showed that T max was decreased by 1.21% in treated OTA samples. On the basis of
reduction in Tmax, it is hypothesized that vaporization temperature of treated OTA sample
was decreased as compared to control which could make it more useful in those
reactions where OTA was used in vapour state. Furthermore, the decrease in
crystallite size and vaporisation temperature may lead to enhance the reaction
kinetics. Therefore, it is assumed that biofield treated OTA could be more
useful as an intermediate in production of various pharmaceutical products. Figure 7: UV spectra of control and treated (T1 and T2)
samples of o-toluic acid. The authors would like to
acknowledge the whole team of Sophisticated Analytical Instrument Facility
(SAIF), Nagpur, Maharashtra and MGV Pharmacy College, Nashik for providing the
instrumental facility. We are very grateful for the support of Trivedi Science,
Trivedi Master Wellness and Trivedi Testimonials in this research work.
1. Lebedev
BL, Pastukhova IV, Eidus YT. Reaction of carbon dioxide with toluene in the
presence of aluminum chloride under pressure (1972) Russ Chem Bull 21: 929-931 2. http://encyclopedia2.thefreedictionary.com/Toluic+Acid. 3. Yang
HJ, Ding MY. Determination of o-toluic acid and its micro amounts of impurities
in industrial products by HPLC (2002) J Liq Chrom Rel Technol 25: 2709-2715 4. Carballo
LM, Wolf EE. Crystallite size effects during the catalytic oxidation of
propylene on Pt/γ-Al2O3 (1978) J Catal 53: 366-373. 5. Chaudhary
AL, Sheppard DA, Paskevicius M, Pistidda C, Dornheim M. Reaction kinetic
behaviour with relation to crystallite/grain size dependency in the Mg–Si–H
system (2015) Acta Mater 95: 244-253. 6. Jain
S, Mills PJ. Biofield therapies: helpful or full of hype? A best evidence
synthesis. (2010) Int J Behav Med 17: 1-16. 7. Movaffaghi
Z, Farsi M. Biofield therapies: biophysical basis and biological regulations?
(2009) Complement Ther Clin Pract 15: 35-37. 8. Giasson
M, Bouchard L. Effect of therapeutic touch on the well-being of persons with
terminal cancer. (1998) J Holist Nurs 16: 383-398. 9. Peck
SD. The efficacy of therapeutic touch for improving functional ability in
elders with degenerative arthritis. (1998) Nurs Sci Q 11: 123-132. 10. Turner
JG, Clark AJ, Gauthier DK, Williams M. The effect of therapeutic touch on pain
and anxiety in burn patients. (1998) J Adv Nurs 28: 10-20. 11. Thomas
AH. Hidden in plain sight: The simple link between relativity and quantum
mechanics (2012) Swansea, UK. 12. http://nccam.nih.gov/health/whatiscam/ 13. Trivedi
MK, Bhardwaj Y, Patil S, Shettigar H, Bulbule A. Impact of an external energy
on Enterococcus faecalis [ATCC-51299] in relation to antibiotic susceptibility
and biochemical reactions-an experimental study (2009) J Accord Integr Med 5:
119-130. 14. Trivedi
MK, Patil S. Impact of an external energy on Staphylococcus epidermis
[ATCC-13518] in relation to antibiotic susceptibility and biochemical
reactions-an experimental study (2008) J Accord Integr Med 4: 230-235. 15. Trivedi
MK, Patil S. Impact of an external energy on Yersinia enterocolitica
[ATCC-23715] in relation to antibiotic susceptibility and biochemical
reactions: An experimental study (2008) Internet J Alternat Med 6: 13. 16. Shinde
V, Sances F, Patil S, Spence A. Impact of biofield treatment on growth and
yield of lettuce and tomato (2012) Aust J Basic Appl Sci 6: 100-105. 17. Sances
F, Flora E, Patil S, Spence A, Shinde V. Impact of biofield treatment on
ginseng and organic blueberry yield (2013) Agrivita J Agric Sci 35: 22-29. 18. Nayak
G, Altekar N. Effect of biofield treatment on plant growth and adaptation
(2015) J Environ Health Sci 1: 1-9. 19. Patil
SA, Nayak GB, Barve SS, Tembe RP, Khan RR. Impact of biofield treatment on
growth and anatomical characteristics of Pogostemon cablin (2012) Biotechnology
11: 154-162. 20. Trivedi
MK, Tallapragada RR. A transcendental to changing metal powder characteristics
(2008) Met Powder Rep 63: 22-28. 21. Dabhade
VV, Tallapragada RR, Trivedi MK. Effect of external energy on atomic,
crystalline and powder characteristics of antimony and bismuth powders (2009)
Bull Mater Sci 32: 471-479. 22. Trivedi
MK, Nayak G, Patil S, Tallapragada RM, Latiyal O. Studies of the atomic and
crystalline characteristics of ceramic oxide nano powders after bio field
treatment (2015) Ind Eng Manage 4: 161. 23. Pavia
DL. Introduction to spectroscopy (2001) Thomson learning, Singapore. 24. Moore
J. Chemistry: The molecular science (2010) Brooks Cole. 25. Trivedi
MK, Patil S, Tallapragada RM. Effect of biofield treatment on the physical and
thermal characteristics of silicon, tin and lead powders (2013) J Material Sci
Eng 2: 125. 26. Morrell
CE, Beach LK. Oxidation of aromatic compounds (1948) U.S. Patent 2443832. 27. Wagner
RB. Preparation of N,N-Diethyltoluamides (1960) U.S. Patent 2932665. 28. Johnson
AW. Invitation to organic chemistry (1999) Jones and Bartlett Publishers,
Burlington, MA, USA. 29. Lang L.
Absorption spectra in the ultraviolet and visible region (1969) Akademiai Kiado
Publishers, Budapest. Characterization of Physical, Thermal and Spectroscopic Properties of Biofield Treated Ortho-Toluic Acid
Abstract
Full-Text
Introduction
Materials and Methods
X-ray diffraction (XRD) study
Differential scanning calorimetry (DSC) study
![]() Where, ΔH Control and ΔH Treated are the
latent heat of fusion of control and treated samples, respectively.
Where, ΔH Control and ΔH Treated are the
latent heat of fusion of control and treated samples, respectively.
Spectroscopic studies
FT-IR spectroscopic characterization
UV-Vis spectroscopic analysis
Results and Discussion
X-ray diffraction
DSC analysis


TGA/DTG analysis
Spectroscopic studies
FT-IR analysis
UV-Vis spectroscopic analysis
Conclusion
Acknowledgement
References