Research Article :
Masood Ayoub Kaloo and Bilal A Bhat To the best of our knowledge, only a few number
of single molecule based receptors synthesized from Diaminomalenonitrile (DAMN)
have been explored and developed as highly selective and sensitive recognition
motifs for cations and anions in an organic,aqueous or mixed solvent system.
The interaction between such receptors and ions has been reported with
remarkable alteration in absorption and fluorescence properties of the molecule.
The signal transduction is prompt, and attributed to the modulation of Internal
Charge Transfer (ICT) and/or Photo Induced Electron Transfer (PET) processes
within the molecule. In this review, we have compiled whole literature about
recognition of ions via DAMN based molecular receptors possessing biological
applications including live cell imaging. Emphasis has been diverted to
summarize and briefly discuss signal response, operation of solvent,
selectivity, binding affinity and detection limit. In addition to this, some of
the crucial design considerations, such as choice of fluorophore, tuning of
electronic effects and mechanism of operation are momentarily discussed Owing
to critical role of ions (cations and anions) in myriad of process of
biological, chemical and environmental significance, considerable attention has
been paid towards the development of single molecule based detection methods
[1,2]. In this direction, a library of cation and anion sensing molecules receptors have been proposed [3,4], and continues to be a key theme in modern
chemistry and biochemistry [5-7]. This can be attributed to the fact that
molecular receptors are highly valuable tools to perform in situ monitoring in
real time, amid high selectivity and sensitivity. Most importantly, synthetic
receptors are usually reusable, easy to operate and realize high sample through
output [8]. A number of interactions, such as hydrogen bonding, electrostatic force,
metal-ligand coordination, hydrophobic and vander Waals forces have been employed
to develop novel and effective receptors [9-11]. Nowadays
the field of molecular recognition has reached a stage where one can
confidently design and synthesize a specialized receptor with a good degree of predictability
and selectivity for many kinds of small or medium-sized ions [12]. In this
direction, use of a wide variety of N-H polarization based molecular
functionalities in the form of amides, ureas, thioureas, ammonium, imidazole,
and Imidazolium based systems have been proposed [13-18]. Besides, chemical
reaction based chemodosimetric and/or coordination approaches utilizing
functional materials with rhodamine, coumarin, BODIPY, hemicyanine,
calixpyrrole, oxazine, naphthalimide, hemicyanine, azo zincon and benzofuran
based molecular units have also been reported [19,20]. However,
in recent times, receptors constructed from DAMN backbone have emerged to be of
significant importance in chemical, biological, and environmental assays. These
molecules besides being robust, exhibit selective response to specific ions or
neutral species. Some of the excellent examples of receptors from DAMN for
various analytes (ions) have been prepared and studied for application in
physiology, medical diagnostics and in imaging. To the best of our knowledge,
no such work has been reported
wherein current state of art of DAMN based receptor platform has been reviewed
along with its biological applications. Thus herein, we have tried to compile
such class of synthetic receptors with biological and live cell imaging
applications. These receptors include Phenothiazine-DAMN, Rhodamine-DAMN,
Azo-azomethine-DAMN, Phenanthroimidazole-DAMN, Triphenyl Schif base-DAMN. Phenothiazine-DAMN:
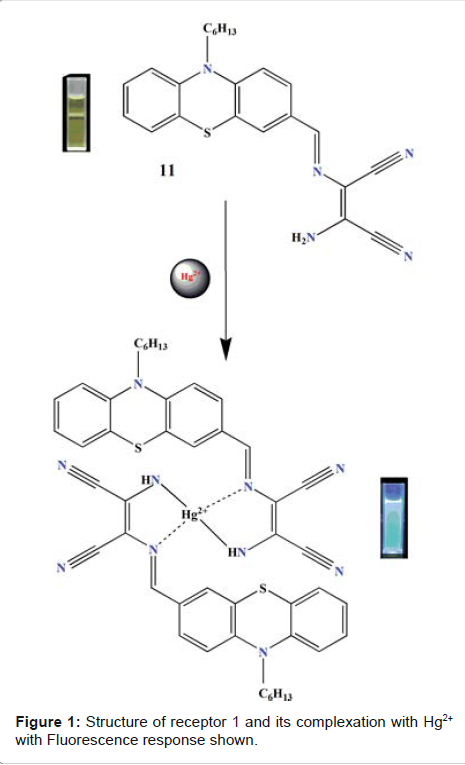
Sekar
et al. studied fluorescent properties of phenothiazine-DAMN synthetic receptor
in the presence of various metal ions in solution (ethanol–water, 6:4,V/V)
[21]. The designed receptor displayed intense emission signal at 550 nm upon
excitation at 425 nm. Upon the addition of Hg2+, quenching of emission band at
550 nm was observed. The results were ascribed due to an ICT (internal charge
transfer) process. Complexation of Hg2+ results in the inhibition of original
ICT process from N-Hexyl Phenothiazine group to the diaminomalenonitrile moiety
(Figure 1). The detection limit (DL) for Hg2+ was found to be 17.8 × 10-9 M.
The binding constant of receptor with Hg2+ was found to be 4.18 x 104 M-1.
Furthermore the probe was also utilized for fluorescence imaging of Hg2+ in
living cells. To test the capability of 1 and explore its imaging application in
living cells, cancer cells were incubated with receptor 1 for 30 min at room
temperate, and then it was treated with HgCl2 for 10 min. The fluorescence
image became dim and quenches, implying that the intracellular uptake of Hg2+
ions complexed with 1 and a yielded non-fluorescent ensemble. Upon further
incubation of cells with S2- (20 μM) for 10 min, green fluorescence image was
recovered, indicating that the uptake of S2- resulted in the decomplexation of intracellular
[receptor + Hg2+] ensemble to fluorescent receptor. Therefore,
the on–off–on fluorescence imaging of receptor was accomplished in cancer
cells. These findings showed that the designed receptor 1 is biocompatible in
nature and can be used fordetecting
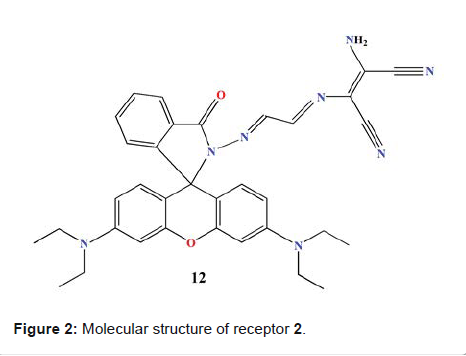
Hg2+ and S2- ions in cells. Rhodamine-DAMN Singaravadivel
et al. reported a novel receptor from Rhodamine dye, conjugated with
diaminomalenonitrile moiety (Molecule 2 in Figure 2). The receptor shows highly
sensitive and selective turn-on
fluorescent response to Cd2+ over other metal ions [22]. Receptor 2 displayed
weak fluorescence response at 553 nm upon excitation at 530 nm. Aft gradual
addition of Cd2+, a significantly strong
emission band at 553 nm appeared. The enhancement, in fluorescence intensity
was likely due to the Cd2+-triggered rhodamine spiroring opening reaction
mechanism. The detection limit of Cd2+ is 18.5 nm in acetonitrile-water (7:3,
V/V). The association constant between receptor and Cd2+ was calculated 2.33 ×
105 M−1. For the live cell imaging via confocal microscopy, HeLa cells
(cervical cancer cells) were used. Receptor 2 possesses low cytotoxicity with
good membrane permeable property and hence is successfully applied to
fluorescence microscopic imaging, for the detection of Cd2+ ions. For live cell
imaging, cells were incubated with a 10 μM solution of receptor 2 for 30 min at
37°C in growth medium, and a very weak fluorescence was observed. Figure 1:
Structure of receptor 1 and its complexation with Hg2+ with Fluorescence
response shown. Figure 2: Molecular structure of receptor 2. In
succession, the cells were added with Cd(NO3)2 (10 μM) for 10 min at 37°C.
After the cells were washed with 3 × 1 mL of PBS three times, an obvious
fluorescence response from the intracellular region was observed. The bright
field image confirmed that the cells were viable throughout the imaging
experiments and the receptor 2 had good cell-membrane permeability, and hence useful
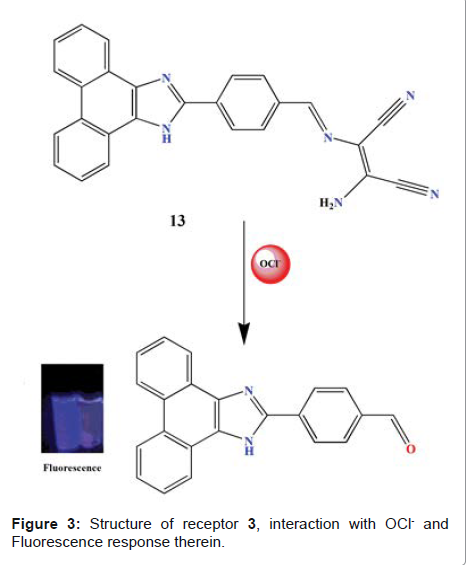
for detecting intracellular Cd2+. Phenanthroimidazole-DMAN In
another study, Zhao et al. reported sensing properties of receptor 3 (Figure 3)
with sodium hypochlorite (NaOCl), and were investigated by means of
fluorescence readout in EtOH/H2O solution (3:1, V/V, 10 mM PBS buffer, pH 7.4)
[23]. The receptor exhibited very weak fluorescence emission upon excitation
wavelength of 340 nm. This is attributed to an efficient ICT from NH2 of DAMN
moiety to phenanthroimidazole (Figure 3). Upon the addition of NaOCl, a
significant enhancement of the emission
intensity at 410 nm was observed. The detection limit for OCl- was found to be 1.4 × 10−2 M. The
imaging experiment showed good cell-membrane permeability of receptor 3, and
was to image exogenous HOCl. The optical window at the blue channel (400–500
nm) was choosen to be a signal output. The HeLa cells (cervical cancer cells)
incubated with receptor (5 M) for 30 min did not display any fluorescence at
400−500 nm. However after addition of OCl− (60 M) and then incubating for
another 30 min, strong fluorescence was clearly observed. In addition to this,
during the experimental process, no obvious cytotoxicity was noticed. Triphynyl
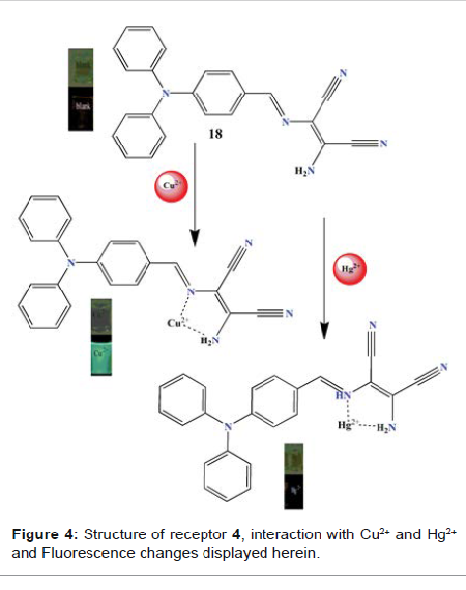
Schiff base-DAMN Zhou
et al. reported the sensing ability of receptor 4 (Figure 4), a Schiff base
derivative based on diaminomalenonitrile, and was tested by mixing it with a
range of metal ions Ag+, Al3+, Ba2+, Ca2+, Ce3+, Co2+, Cr3+, K+, La3+, Li+,
Mg2+, Na+, Ni2+, Pb2+, Zn2+, Fe3+, Hg2+ and Cu2+ in acetonitrile solvent [24].
Fluorescence spectra showed that only Cu2+ results in a pronounced fluorescence
enhancement at 501 nm. In the absorption spectroscopy, signal at 420 nm
decreases and a new band centred at 350 nm with blue shift 70 nm emerged. The
phenomenon was attributed to the fact that the magnitude of the energy
difference between the ground and excited states of a dipolar compound is
significantly influenced by intermolecular solute-solvent interactions.
Increased dipoledipole interactions between the receptor and polar solvents lead
to the lowering of energy levels. The color of the solution changes from pale
yellow to colourless (Figure 4) which can be attributed to the Metal-Ligand
(ML) type electronic transition. The detection limit of Cu2+ in molar fraction
is 0.5. To evaluate the performance of receptor with Cu2+ and receptor alone in
living cells, the fluorescence microscopy imaging was performed. HepG2 cells
(carcinoma cells derived from liver), testing candidates were cultured and
stained with the sample for 2h. The excess substances were washed away by
buffer solution and the fluorescent signal was collected by using confocal
laser scanning microscopy. The mixture of Cu2+ with receptor 4 show good cell
imaging ability. This could be attributed to the fact that addition of Cu2+
ions not only result in enhancement
of the fluorescence intensity of the solution, but also increased the aqueous
solubility of the mixtures. The fluorescent and the merged images showed that
the mixtures go through the membrane and just localize uniformly in the
cytoplasm. The intense fluorescence is mainly because the mixture internalizes
in Figure 3: Structure of receptor 3, interaction with OCl- and Fluorescence
response therein. Figure 4: Structure of receptor 4, interaction with Cu2+ and
Hg2+ and Fluorescence changes displayed herein. the HepG2 cell cytoplasm and
the distribution in the nucleolus is significantly
lower, suggesting that only the cell cytoplasm can be labelled by the mixture.
The results demonstrate the bioimaging application of receptor 4 for Cu2+ ions. Azo-azomethine-DAMN Khanmohammadi
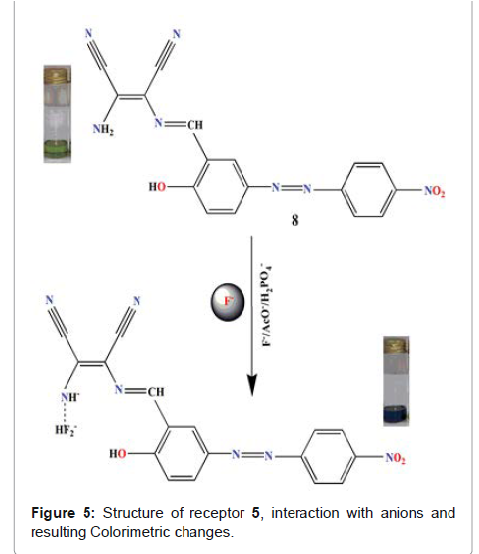
et al reported receptor (5 in Figure 5), a N-monosubstituted
diaminomalenonitrile-based azo-azomethine dye for the anion-recognition with
tetrabutylammonium (TBA) salts of F, AcO- and H2PO4 - in (9:1, V/V: DMSO-water)
solvent system [25]. Here progressive addition of F¯ resulted in the red shift
of 94 nm accompanied by the dramatic color change from light green to greenish
blue. The colour change could be attributed to the deprotonation of receptor at
-NH2 site. This phenomenon results
in the concentration of negative charge on the nitrogen which results in the
enhancement of the push–pull effect of the ICT transition. Here the
introduction of electron-withdrawing group (NO2) decreases the electron density
on nitrogen atomsand hence increases the acidity of hydrogen-bind donors, so
the deprotonation becomes easily. The detection limit of F¯, AcO¯ and H2PO4¯
ions was found 1.50×10-6, 1.21×10-6 and 2.25×10-6 M respectively. The
association constants for receptor towards F¯, AcO¯ and H2PO4¯ were calculated
to be 1.59×105, 1.35×105 and 1.54×105 M-1 respectively. The designed receptor
was used for qualitative and quantitative detection of inorganic fluoride in toothpaste
and mouthwash. Due to the conspicuous color change from light green to greenish
blue the current receptor delivers immense potential to detect fluoride even by
the naked eye in the above samples. This
review summarizes the foremost progress made in the development of molecular
receptors designed from DAMN backbone. On this functionality, large numbers of
synthetic receptors have been described for the recognition of a range of anions
and cations (F-, Cu2+, OCl-, Cd2+, S-, Hg2+ and AcO-) in both organic as well
as mixed solvent system (organic/aqueous). The proposed receptor platform
displayed pronounced colorimetric changes and/or fluorescence turn on response with the ions asa function of concentration. Besides these, the
proposed receptor design is highly robust and displays sensing of ions in vitro
as well as vivo. Because of such crucial characteristics, we have summarized here
only those receptor which display applications for biological samples. Some of
the crucial design considerations, such as choice of fluorophore/chromophore,
selectivity, application in bio systems and mechanism of recognition are
momentarily discussed. The detection limit as well as association/binding
constant of the discussed receptors is also herewith. The recognition signal
has been proposed to occur via the interaction between receptor with the ion,
and hence resulting in the modulation of ICT and/or PET processes. We
highly acknowledge department and lab staff of chemistry at IUST for their
support during various stages throughout the work. M. A. Kaloo gratefully
acknowledges Department of Science and Technology, New Delhi for
INSPIRE-FACULTY award [DST/INSPIRE/04/2016/000098] and allied research grants. 1.
H.N. Kim, W.X. Ren, J.S. Kim and J. Yoon. Fluorescent and colorimetric sensors
for detection of lead, cadmium, and mercury ions (2012) Chem Soc Rev 41: 3210.Current State of Art of Diaminomalenonitrile Based Synthetic Receptors for Ion Sensing With Bio Applications: Mechanistic, Sensing and Photophysical Aspects
Abstract
Full-Text
Introduction
Conclusions
Acknowledgements
References
2.
B. E. Kim, T. Nevitt and D. J. Thiele. Mechanisms for copper acquisition,
distribution and regulation. (2008) Nat Chem Biol 4: 176.
3.
R. Uauy, M. Olivares and M. Gonzalez. Essentiality of copper in humans (1998)
Am J Clin Nutr 67: 952.
4. D
Karak, S Lohar, A Banerjee, A Sahana, I Hauli,et al. Interaction of soft donor
sites with a hard metal ion: crystallographically characterized blue emitting
fluorescent probe for Al(III) with cell staining studies (2012) RSC Adv 2:
12447.
5.
P. de Silva, D. B. Fox, A. J. M. Huxley and T. S. Moody, Coord. Progress in
Heterocyclic Chemistry: A Critical Review of the 2000literture (2000) Chem Rev
205: 41.
6.
R. Martı nez, A. Espinosa, A. Ta rraga and P. Molina. Bis(indolyl) methane
derivatives as highly selective colourimetric and ratiometric fluorescent molecular
chemosensors for Cu2+cations(2008)
Tetrahedron 64: 2184.
7.
AP de Silva, AJM Huxley and TS Moody. Combining luminescence, coordination and
electron transfer for signalling purposes (2000).Coord Chem Rev 205: 41.
8.
V. Amendola, L. Fabbrizzi. Anion receptors that contain metals as structural
units (2009) Chem Commun 513.
9. A
Ajayaghosh, P Carol, S Sreejith. A Ratiometric Fluorescence Probe for Selective
Visual Sensing of Zn2+ (2005) J Am ChemSoc 127: 14962.
10.
D Wang, Y Ke, H Guo, J Chen, W Weng. A novel highly selective colorimetric
sensor for aluminum (III) ion using Schiff base derivative (2014) Spectrochim.
Acta, Part A 122: 268.
11.
V Amendola, L Fabbrizzi, M Licchelli, C Mangano, P Pallavicini, Et al.
Molecular events switched by transition metals (2000) Coord Chem Rev 192: 649.
12.
AP Silva, HNQ Gunaratne, T Gunnlaugsson, AJM Huxley, CP McCoy, et al. Signaling
Recognition Events with Fluorescent Sensors and Switches (1997) Chem Rev 97:
1515.
13.
P. D. Beer and P. A. Gale. Anion Recognition and Sensing: The State of the Art
and Future Perspectives (2001) Angew Chem 40:486.
14.
C. Perez-Casas and A. K. Yatsimirsky, J. Detailing Hydrogen Bonding and
Deprotonation Equilibria between Anions and Urea/Thiourea Derivatives (2008)
Org Chem 73: 2275.
15.
FY Wu, ZC Wen, N Zhou, YF Zhao, YB Jiang. A novel thioureabased dual
fluorescent anion receptor with a rigid hydrazine spacer (2002) Org Lett 4:
3203.
16.
J. M. Linares, D. Powell and J. K. Bowman, Coord. Chem. Rev., 2003, 240, 57.
17.
X. J. Peng, Y. K. Wu, J. L. Fan, M. Z. Tian and K. L. Han, J. Org.Chem., 2005,
70, 10524.
18.
Chellappan, N. J. Singh, I. C. Hwang, J. W. Lee and K. S. Kim. A calix[4]imidazolium[2]pyridine
as an anion receptor (2005) Angew Chem 44: 2899.
19.
L. Yang, X. Li, J. Wang, Y. Qu and J. Hua. Colorimetric and Ratiometric
Near-Infrared Fluorescent Cyanide Chemodosimeter Based on Phenazine Derivatives
(2013) ACS Appl Mater Interfaces 5: 1317.
20.
J Ren, W Zhu, H Tian. A highly sensitive and selective chemosensor for cyanide
(2008) Talanta 75: 760-764.
21.
SR Patil, AS Choudhary, N Sekar. A Lawsone–DAMN based colorimetric chemosensor
for rapid naked-eye detection of mercury(II) (2016) New J. Chem 40: 6803-6811.
22.
P. Sakthivel, K Sekar, G Sivaraman, S Singaravadivel, J Fluoresc. Rhodamine
Diaminomaleonitrile Conjugate as a Novel Colorimetric Fluorescent Sensor for
Recognition of Cd2+ Ion (2017) J Fluoresc 27:
1109-1115.
23.
Y Zhao, H Li, Y Xue, Y Ren, T Han. A phenanthroimidazole-based fluorescent
probe for hypochlorous acid with high selectivity and its bio-imaging in living
cells (2017) Sensors and Actuators B 241: 335–341.
24.
W Xi, Y Gong, B Meic, X Zhang, Y Zhang, et al. (2014) Sensors and Actuators B
14: 1045-1068.
25. H. Khanmohammadi, K. Rezaeian, A. Abdollahi. Colorimetric detection of anions
in aqueous media using N-monosubstituted diaminomaleonitrile-based
azo-azomethine receptors: real-life applications (2014) Spectrochimica Acta
Part A: Molecular and Biomolecular Spectroscopy 14: 1868-1898. Keywords