Research Article :
Fred H Previc, Ruth A Ross and Gregg Siegel The relationship between topographical and
non-topographical cognitive measures was studied for 25 elderly participants.
The topographical measures were the Camden Topographical Recognition Memory
Test (CTRMT), a Topographical Mental Rotation Test (TMRT), and a Virtual Pond
Maze (VPM). The non-topographical tests were the Montreal Cognitive Assessment
(MoCA), the Trail-Making Test-B (TMT-B), and a matching-to-sample Visual
Short-Term Memory Test (VSMT). Only the correlation (0.48) between the TMT-B
and the TMRT attained significance; the bivariate correlations among the three
topographical measures were modest, ranging from 29 to 33, although they did
correlate highly with a topographic composite score (69-78). A factor analysis
yielded a further distinction between the topographical and non-topographical
measures. Loadings for the three topographical measures on a presumed topographical
factor ranged from 0.62 to 0.71 but only from 0.17 to 0.23 for the second
factor; the MoCA and TMT-B loaded on both factors, while the VSMT measure
loaded poorly (-0.03) on the topographical factor but highly (0.89) on the
second factor. The results suggest that standard measures of cognitive function
may not be optimal for specific assessment of topographical abilities, the best
predictor of impending Alzheimers dementia. The
topographical orientation system, also known as the spatial navigation, topo-kinetic
and action-extrapersonal systems, is one of the four major networks in the
brain governing our interaction with our 3D environment [1]. It is believed to
be composed mainly of subcortical regions such as the caudate nucleus and anterior
thalamus and three posterior regions-the medial-temporal lobe/hippocampus,
posterior cingulate, and parietal-temporal cortex [1-3]. It is the system
responsible for scene and route memory, a sense of presence in the world, and
topographical orientation and way finding in the plane of the Earths surface
[1]. It is widely accepted that the topographical memory component of this system is
centered in the hippocampus. Activation of the hippocampus occurs during recall
of topographical routes [4] whereas damage to it results in amnesia for spatial
landmarks and maps and severe topographical disorientation [5]. Although the
topographical memory system deteriorates during normal aging [6-8], severe disruption
of it is a very early diagnostic sign of Alzheimers
disease [8-15]. Indeed, atrophy and/or metabolic deactivation of key
components of the topographical neural network are among the best predictors of
impending Alzheimers disease [16-19]. Despite their neurophysiological basis and good predictive validity,
topographical tests are not routinely used in assessment of early Alzheimers
disease. The
purpose of the present study, therefore, was to compare topographical versus
more widely used non-topographical measures of cognitive ability in older
adults. To test topographical memory, three tests were used in the present
study: 1.
The Camden Topographical Recognition Memory Test (CTRMT) 2.
A computerized Topographical Mental Rotation Test (TMRT) similar to the “four
mountains” test used by Bird et al. [9] and Hartley and Harlow [20]. 3.
A VirtualPond Maze (VPM) similar to the widely used Morris water maze in rodents and
virtual mazes that have been developed for humans [21-23]. While the CTRMT has
not been specifically linked to hippocampal function, memory for distinct
scenes is dependent on this region [24,25]. Memory for scenes requiring
topographical mental rotation is also dependent on the hippocampus and the
critical role of the
hippocampus in the water maze has been repeatedly shown in both animals and
humans [20-22].
The tests of non-topographical function were the Montreal Cognitive Assessment
(MoCA), the Trail-Making Test Part B (TMT-B), and a computerized Visual
Short-Term Memory Task (VSMT) that had a similar appearance to the TMRT except
that it involved a matching-to-sample object recognition memory rather than a
topographical transformation. Even though it was a basis for screening of the
participant population, the MoCA ended up replacing a different non-topographical
test-the Camden Short Recognition Memory Test for Words-because scores on the
latter severely violated normality due to a ceiling effect. To
determine relationships within and across the two sets of measures, a biserial
correlation matrix and factor analysis were performed on scores from the six
measures. Participants A
total of 25 active individuals between the ages of 70 and 85 participated in
this study. All participants were prescreened for dementia and those scoring
less than 19 on the MoCA-the recommended cutoff for the normal designation for a
similar age sample-were excluded from the study [26]. All but two of 27
originally recruited participants that met other exclusionary criteria achieved
the MoCA criterion. Participants were additionally excluded if they had ever
suffered a stroke, seizure, traumatic brain injury, or had a diagnosed neurologicalcondition. Finally, participants were excluded if they were currently
taking any psychoactive drugs and engaged in other than moderate caffeine
consumption (no more than 300 mg per day, the equivalent of three cups of
coffee per day), moderate alcohol consumption, and mild pain medication (used
by two participants). Participants signed an informed consent document approved
by Biomed IRB (Biomedical Institute of America; San Diego, CA). Apparatus All
cognitive
testing was performed in an outpatient office setting and required
approximately 90 min. Two of the three topographical tests (the VPM and TMRT)
were programmed for this study and administered using a Compaq NC6400 computer
(Hewlett-Packard) under moderately dim illumination in a quiet room while the
participant was seated. The third topographical test was the CTRMT,
administered from a test booklet using standardized procedures in a well-lit
room [27]. Camden
Topographical Recognition Memory Test (CTRMT): This
task required participants to view 30 urban scenes during a 3-s interval and
report whether the photograph was taken by an amateur or a professional
photographer. Immediately after the completion of the presentation sequence,
participants were shown the same 30 scenes in a different order along with two
never-before-seen versions of each scene taken from different distances and egocentric
viewing perspectives. In a three-alternative forced-choice recognition
procedure with self-pacing, participants were then required to point to the
scene that was originally presented. Each score was based on the total correct
out of 30. Topographical Mental Rotation Test
(TMRT): Participants viewed a scene containing a set of three objects
(e.g., red cylinder, blue sphere, green cube) and were then shown a figure
instructing them to rotate their viewpoint 90º left, 90º
right, or 180º opposite (see Figure 1, left panel). They were then
shown three scenes from each of the rotated viewpoints and asked to click on
the image that depicted the correct viewpoint shift. A yellow box indicated the
participants choice while a green box (appearing only during practice trials)
showed the correct viewpoint. Participants had 12 s to view each scene and 14 s
to make their response, with a timer appearing during the last 5 s of the
forced-choice interval. After being presented with instructions, which included
demonstrations of the task using actual objects, participants viewed a set of
12 practice trials (they could view a second set if they chose to) and then
were presented with 15 test trials. Each score was based on the total number of
correct responses out of 15. Montreal
Cognitive Assessment (MoCA): The MoCA consists of 30 points derived from a set
of 12 tasks, including a miniature version of the trail-making task (1 pt), a
Necker cube copy (1 pt), clock drawing (3 pts), naming (3 pts), delayed recall
(5 pts), forward and reverse digit span (2 pts), go/no-go attention (1 pt),
serial-seven subtraction (3 pts), sentence repetition (2), verbal fluency (1
pt), verbal analogous reasoning (2 pts), and general orientation (6 pts) (see www.mocatest.org ) Testing on the
MoCA
required about 10 min [29]
Trail-Making Test Part B (TMT-B): The
TMT consists of two tasks: Part A, which presents a connect-the-dots sequencing
and visual scanning task for numbers only; and Part B, which requires alternate
sequencing of numbers and letters. Each task is preceded by a sample that provides
for training and practice. The dependent variable was time to completion, with
timing beginning immediately after the participant began to move the pencil. Errors
were corrected immediately by returning the participant to the last correct
point in the sequence, in accordance with standardized administration
procedures. Only time to completion for Part B was included in the final
analysis. Visual Short-Term Memory Test (VSMT):
This task was designed as a non-topographical comparison to the TMRT.
The stimuli and grid were identical to the TMRT task shown in Figure 1, except
that four objects were presented rather than three. However, the task differed
in that the viewing perspective and spatial locations of the objects never
changed during the testing phase; rather, the three test stimuli consisted of
the original scene and two scenes in which one of the four objects in the
original scene changed its shape or color (but not both). In the two discrepant
scenes, the same object could change in different ways (e.g., a red sphere
could become a red cube in one scene or a blue sphere in the
other) or different objects could change in the same (both color changes) or
different ways (e.g., a blue sphere could change to a green sphere in one scene
while a yellow cone could turn into a yellow cube in the other). Participants
had 9 s to view each scene and 12 s to make their response, with a timer
appearing during the last five seconds of the forced-choice interval. As in the
TMRT, participants were required to click on the original image during the
recognition period, which caused a yellow box to appear over their selection
while a green box surrounded the correct response (green box appeared only
during practice trials). After being presented with instructions, participants
viewed a set of 12 practice trials (they could view a second set if they chose
to) and then were presented with 15 test trials. Each score was based on the
total number of correct responses out of 15. Figure 1: An illustration of the stimuli used in the TMRT task (left) and pond maze (right). Analysis A
total of six variables were analyzed in this study: CTRMT
(# correct), TMRT (# correct), VPM (time to completion), MoCA (# correct),
TMT-B (time to completion), and VSTMT (# correct). SPSS (IBM, Chicago, IL) was
used to perform an exploratory factor analysis on the data using a varimax
rotation and Kaiser Normalization. Because higher scores (times) on the VPM and
TMT-B reflected poorer performance whereas higher scores on the other four
tests reflected better performance, time to completion scores on the VPM were
subtracted from the cutoff time of 60 s whereas times on the TMT-B were
subtracted from the maximum time in the study (164 s). Longer times on the VPM
and TMT-B now reflected better performance, without otherwise transforming the
data; hence, the correlation between the actual and inverted times was -1.0 for
both measures. A
“composite” topographic measure was created by turning the three raw
topographic scores for each participant into percentiles, based on established
norms (in the case of the CTRMT) or the present studys means and standard
deviations (for the VPM and TMRT scores), and then averaging all three
together. The
sample consisted of 16 females and nine males, with an average age of 76.4
years. Nine of the 25 participants were Hispanic, 12 were non-Hispanic
Caucasians, three were Asian-Americans, and one self-described as “other”. In
terms of education level, seven had high-school degrees, six had college
degrees, and 12 had graduate degrees. The
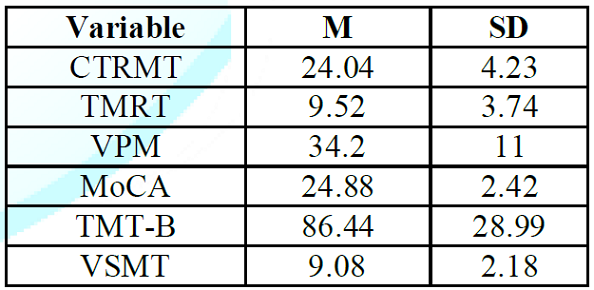
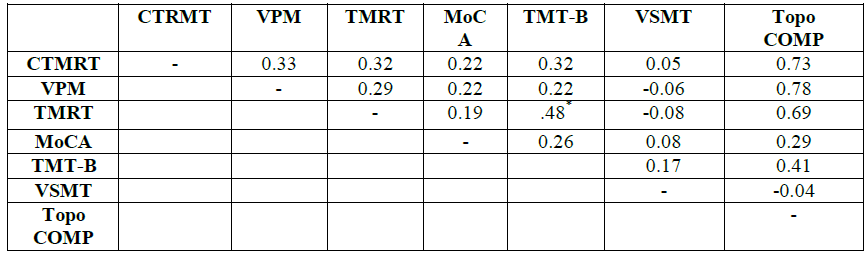
means and standard deviations for the six cognitive measures are shown in (Table 1). The complete correlation
matrix containing all 21 bivariate correlations is shown in (Table 2), while the results of the
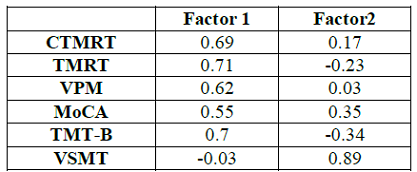
exploratory factor analysis are shown in (Table
3).
Table 1: Means and
Standard Deviations for the Topographical and Non-topographical Measures.
On
the tests in which number of correct was measured, mean performance ranged from
slightly over 60% (TMRT and VSMT) to slightly over 80% (MoCA and CTMRT). The
average score on the CTRMT, MoCA and TMT-B
tests were similar to age-adjusted norms from previous studies [26, 30-31]. Many
participants had trouble with the VPM, TMRT, and VSMT, either exceeding the
cutoff time in the case of the VPM or scoring at chance levels or below on the
TMRT and VSMT. The largest variability (as a percentage of the mean) occurred in
the TMRT test, partly because of a large (>50%) gender difference, with
males averaging 12.44 correct and females averaging 7.8 correct. No other
measure yielded a gender difference of more than 15%. The
bivariate correlation matrix is shown in Table 2. Because scores on all but one
of the six measures violated normality according to the Shapiro-Wilk statistic,
Spearmans rho was used to determine all correlations. Table 3: Factor Loadings
on Principal Components.
Only one correlation-that between the TMRT and TMT-B scores-proved significant,
with the correlations among the three topographical measures ranging from 0.29
to 0.33. The correlations between each of the three topographic measures and
the topographical composite score ranged from 0.69 to 0.78; these high
correlations would be expected in that each of the topographic scores
contributed a third to the composite score. The correlations between the
topographical composite and the non-topographic scores were much more modest,
the highest (0.41) being the TMT-B-composite one.
The results of the factor analysis revealed two principal components with
eigenvalues greater than 1.0 that accounted for 54.6% of the variance (Factor
1=36.1%; Factor 2=18.5%). The loadings for each of the six variables on each
factor are shown in Table 3. The three topographical variables loaded very
highly on Factor 1 (0.69, 0.62. and 0.71, for the CTMRT, VPM, and TMRT
measures, respectively) but loaded poorly on Factor 2 (0.17, 0.03, and -0.23,
for those same variables). Hence, it may be concluded that Factor 1 is related
to topographical abilities. The TMT-B also loaded highly on Factor 1 (0.70) and
the MoCA moderately no (“0.55), while the VSMT loaded poorly (-0.03). Conversely,
the three non-topographic measures loaded better than the topographic ones on
Factor 2. To
illustrate further the dissociation between the topographical and non-topographical
measures, MoCA scores for the four participants with the highest topographical
composites (range=23-27, M=25.5) overlapped the MoCA
scores for the four participants with the lowest topographical composites
(range=19-27, M=23.25). In fact, the participants with the two lowest
topographical composites (6th and 16th percentile) scored near- and
above-average on the MoCA (23 and 27, respectively, relative to the sample mean
of 24.88). The
results of this preliminary study show dissociation between topographical and
non-topographical measures of cognitive function. Despite some limitations of
this study that will be addressed, this result could hold potentially important
implications for future screening for mild cognitive impairment and early Alzheimers
dementia.
The two principal components derived from the factor analysis distinguished the
topographical measures from the non-topographical measures. The topographical
memory measures all loaded highly on the first factor (>.6) but poorly on
the “non-topographical” factor (<.25). The reverse was true for the visual
memory test, which loaded highly on the second factor (.89) but poorly on the “topographical”
factor (-.03). The TMT-B also loaded very well on the “topographical” factor (.70)
and the MoCA somewhat (.55), but they also loaded more strongly than the
topographical measures on the second factor, which presumably involved non-topographical
memory ability. The results of the factor analysis must be viewed with caution,
however, given that the sample size was relatively small and none of the
bivariate correlations between the topographical measures proved statistically
significant.
It is unclear why the bivariate correlations among the topographical memory
measures were so modest, given their loadings on the “topographical” factor. Although
the three topographical memory measures have been shown to tap into hippocampal
function [9, 20-22, 24-25], it is likely that they represent slightly different
cognitive processes. It has previously been shown that topographical memory and
active navigation are not always well correlated [10]. Moreover, the gender
bias only in the TMRT suggests that it additionally reflects some of the same
spatial rotational processes previously shown to be superior in males [32],
possibly involving the parietal-temporal portion of the topographical memory
system. It may very well be the case that poor performance on a single
topographical test is of less clinical significance than a global topographical
impairment, as assessed by a composite topographical score.
The partial dissociation between the topographical and non-topographical
measures in this study has potentially important implications for screening for
Alzheimers disease. Although widely used measures of dementia such as the MoCA
and TMT-B may tap into topographical abilities and even hippocampal function in
the latter case, they are also dependent on other brain areas such as the
frontal lobes, which fail later in the time-course of Alzheimers disease. [33,34].
The TMT-B requires cognitive shifting and other executive functions, while the
MoCA is mostly composed of verbal and working-memory items. While the TMT-B can
discriminate the mildly cognitively impaired from healthy controls [11,35], it
may not be as sensitive as pure topographical measures in diagnosing the
initial topographical deficit in Alzheimers
[11,15].
Of the topographical tests, the CTRMT may be the most advantageous in that it
is standardized, normed, easy to administer, and achievable by all
participants, in addition to loading highly on composite “topographical” factor
measures. We
wish to acknowledge the assistance of Sam Washburn (computer programming), Dr.
Michael Roman (neuropsychological testing), Dr. Dora Angelaki (experimental
protocol), John Hatch (statistics) and Karl McCloskey (administrative). Research
reported in this publication was supported by the National Center for Advancing
Translational Research (NCATS); the National Institute on Aging (NIA); and the
National Heart, Lung and Blood Institute (NHLBI) under Award #TR000645-01. The
content is solely the responsibility of the authors and does not necessarily
represent the official views of the National Institutes of Health. 1.
Previc
FH. The neuropsychology of 3-D space (1998) Psychol Bull 124: 123-64. https://psycnet.apa.org/doi/10.1037/0033-2909.124.2.123 2.
Berthoz
A. Parietal and hippocampal contribution to topokinetic and topographic memory (1997)
Philos T Roy Soc B 352: 1437-1448. https://doi.org/10.1098/rstb.1997.0130 3.
Maguire
EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, et al. Knowing where and
getting there: A human navigation network (1998) Science 280: 921-924. https://doi.org/10.1126/science.280.5365.921 4.
Maguire
EA, Frackowiak RS and Frith CD. Recalling routes around London: activation of
the right hippocampus in taxi drivers (1997) J Neurosci 17: 7103-7110. https://doi.org/10.1523/jneurosci.17-18-07103.1997 5.
Aguirre
GK and DEsposito M. Topographical disorientation: A synthesis and taxonomy (1999)
Brain 122: 1613-1628. https://doi.org/10.1093/brain/122.9.1613 6.
Adamo
DE, Briceno EM, Sindone JA, Alexander NB and Moffat SD. Age differences in
virtual environment and real world path integration (2012) Front Aging Neurosci
4: 26. https://doi.org/10.3389/fnagi.2012.00026 7.
Moffat
SD, Elkins W and Resnick SM. Age differences in the neural systems supporting
human allocentric spatial navigation (2006) Neurobiol Aging 27: 965-972.
https://doi.org/10.1016/j.neurobiolaging.2005.05.011 8.
Monacelli
AM, Cushman, LA, Kavcic V and Duffy CJ. Spatial disorientation in Alzheimers
disease: The remembrance of things passed (2003) Neurology 61: 1491-1497.
https://doi.org/10.1212/wnl.61.11.1491 9.
Bird
CM, Chan D, Hartley T, Pijnenburg YA, Rossor MN, et al. Topographical
short-term memory differentiates Alzheimers disease from frontotemporal lobar
degeneration (2010) Hippocampus 20: 1154-1169. https://doi.org/10.1002/hipo.20715 10.
Boccia
M, Di Vita, A, Diana, S, Margiotta, R, Imbriano L, et al. Is losing ones way a
sign of cognitive decay? Topographical memory deficit as an early marker of
pathological aging (2019) J Alzheimers Dis 68: 679-693. https://doi.org/10.3233/jad-180890 11.
Chan
D, Gallaher LM, Moodley K, Minati L, Burgess N, et al. The 4 Mountains test: A short
test of spatial memory with high sensitivity for the diagnosis of pre-dementia
Alzheimers disease (2017) J Visual Exp 116: 54454. https://doi.org/10.3791/54454 12.
Guariglia
CC and Nitrini R. Topographical disorientation in Alzheimers disease (2009) Arq
Neuropsiquiatr 67: 967-972. https://doi.org/10.1590/s0004-282x2009000600001 13.
Pengas
G, Patterson K, Arnold RJ, Bird CM, Burgess N, et al. Lost and found: Bespoke
memory testing for Alzheimers disease and semantic dementia (2010) J Alzheimers
Dis 21: 1347-1365. https://doi.org/10.3233/jad-2010-100654
14.
Previc
FH. Vestibular loss as a contributor to Alzheimers disease (2013) Med
Hypotheses 80: 360-367. https://doi.org/10.1016/j.mehy.2012.12.023 15.
Wei
EX, Oh ES, Harun A, Ehrenburg M and Agrawal Y. Vestibular loss predicts poorer
spatial cognition in patients with Alzheimers disease (2018) J Alzheimer Dis 61:
995-1003. https://doi.org/10.3233/jad-170751 16.
Huang
C, Wahlund LO, Svensson L, Winblad B and Julin P. Cingulate cortex
hypoperfusion predicts Alzheimers disease in mild cognitive impairment (2002)
BMC Neurol 2: 9. https://doi.org/10.1186/1471-2377-2-9 17.
Johnson
NA, Jahng GH, Weiner MW, Miller BL, Chui HC, et al. Pattern of cerebral
hypoperfusion in Alzheimer disease and mild cognitive impairment measured with
arterial spin-labeling MR imaging: Initial experience (2005) Radiology 234: 851-859.
https://doi.org/10.1148/radiol.2343040197 18.
Lithfous
S, Dufour A and Després O. Spatial navigation in normal aging and the prodromal
stage of Alzheimers disease: Insights from imaging and behavioral studies (2013)
Ageing Res Rev 12: 201-213. https://doi.org/10.1016/j.arr.2012.04.007 19.
Mosconi
L. Brain glucose metabolism in the early and specific diagnosis of Alzheimers
disease. FDG-PET studies in MCI and AD. (2005) Eur J Nucl Med Mol I 32: 486-510.
https://doi.org/10.1007/s00259-005-1762-7 20.
Hartley
T and Harlow R. An association between human hippocampal volume and
topographical memory in healthy young adults (2012) Front Human Neurosci 6: 338.
https://doi.org/10.3389/fnhum.2012.00338 21.
Sharma
S, Rakoczy S and Brown-Borg H. Assessment of spatial memory in mice. Life Sci
(2010) 87: 521-536. https://doi.org/10.1016/j.lfs.2010.09.004 22.
Brandt
T, Schautzer F, Hamilton DA, Bruning R, Markowitsch HJ, et al. Vestibular loss
causes hippocampal atrophy and impaired spatial memory in humans (2005) Brain
128: 2732-2741. https://doi.org/10.1093/brain/awh617 23.
Moffat
SD and Resnick SM. Effects of age on virtual environment place navigation and
allocentric cognitive mapping (2002) Behav Neurosci 116: 851-859. https://doi.org/10.1037//0735-7044.116.5.851 24. Epstein R
and Kanwisher N. A cortical representation of the local visual environment
(1998) Nature 392: 598-601. https://doi.org/10.1038/33402 24.
Epstein
R and Kanwisher N. A cortical representation of the local visual environment
(1998) Nature 392: 598-601. 25.
Bonnici
HM, Kumaran D, Chadwick MJ, Weiskopf N, Hassabis D, et al. Decoding
representations of scenes in the medial temporal lobes (2012) Hippocampus 22: 1143-1153.
https://doi.org/10.1002/hipo.20960 26.
Trzepacz
PT, Hochstetler H, Wang S, Walker B, Saykin AJ, et al. Relationship between the
Montreal Cognitive Assessment and Mini-mental State Examination for assessment
of mild cognitive impairment in older adults (2015) BMC Geriatr 15: 107. https://doi.org/10.1186/s12877-015-0103-3 27.
Warrington
E. The Camden memory tests manual (1996) East Sussex, England: Psychology
Press, UK 28.
Previc
FH, Krueger WW, Ross RA, Roman MA and Siegel G. The relationship between
vestibular function and topographical memory in older adults (2014) Front
Integr Neurosci 8: 1-8. https://doi.org/10.3389/fnint.2014.00046 29.
Nasreddine
ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, et al. The Montreal
Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment
(2005) J Am Geriatr Soc 53: 695-699. https://doi.org/10.1111/j.1532-5415.2005.53221.x 30.
Lee
DM, Tajar A, Ulubaev A, Pendleton N, ONeill TW, et al. The association between
different cognitive domains and age in a multicentre study of middle-aged and
older European men (2009) Int J Geriatr Psych 24: 1257-1266. https://doi.org/10.1002/gps.2255 31.
Tombaugh
TN. Trail Making Test A and B: Normative data stratified by age and education
(2004) Arch Clin Neurol 19: 203-214. https://doi.org/10.1016/s0887-6177(03)00039-8 32.
Nazareth
A, Herrera A and Pruden SM. Explaining sex differences in mental rotation: Role
of spatial activity experience (2013) Cogn Process 14: 201-204. https://doi.org/10.1007/s10339-013-0542-8 33.
Leirer
VM, Wienbruch C, Paul-Jordanov I, Kolassa S, Elbert T, et al. Hippocampal
activity during the transverse patterning task declines with cognitive
competence but not with age (2010) BMC Neurosci 11: 113. https://doi.org/10.1186/1471-2202-11-113 34.
Stuss
DT, Bisschop SM, Alexander MP, Levine B, Katz D, et al. The Trail-Making Test: A
study in focal lesion patients (2001) Psychol Assessment 13: 230-239. https://doi.org/10.1037//1040-3590.13.2.230 35.
Ashendorf
L, Jefferson AL, OConnor MK, Chaisson C, Green RC, et al. Trail making test
errors in normal aging, mild cognitive impairment, and dementia (2008) Arch
Clin Neuropsych 23: 129-137. https://doi.org/10.1016/j.acn.2007.11.005 Previc FH, Biomedical Development Corporation, 620 East Dewey Place, San
Antonio 78212, Texas, USA, Tel: +612434014044, E-mail: fprevic@sbcglobal.net Previc FH, Ross
RA and Siegel G. Dissociation of measures of topographical and
non-topographical cognitive ability in older adults (2019) Neurophysio and
Rehab 2: 47-51 Topographical, Memory, Hippocampus, Elderly,
Alzheimers.Dissociation of Measures of Topographical and Non-topographical Cognitive Ability in Older Adults
Abstract
Full-Text
Introduction
Methods
The three non-topographical tests were the MoCA (which includes a clock-drawing
and a Necker-Cube copying task but no topographical assessment per se), the
TMT-B, and a computerized short-term visual memory test. The MoCA and the TMT-B
were administered as paper-and-pencil tests, while the VSMT was programmed for
this study and also administered on the Compaq NC6400 computer. These tests
were carried out as part of a larger study that also analyzed several measures
of vestibular function and their relationship to topographical abilities with
vestibular testing performed in a separate facility [28].Tests
Virtual Pond Maze (VPM): Participants
started from one of six locations around the virtual pond, surrounded by six
houses and six trees, all evenly spaced such that each starting location
provided a unique set of spatial landmarks (see Figure 1, right panel). Using only the left and right cursor
arrows, participants were required to navigate to a fixed platform slightly
offset from the middle of the pond, with the forward speed set by the computer
at a simulated 7 m/s. The location and size of the platform was set such that
participants had to make at least one cursor correction to reach the platform
from each of the starting points. A cutoff value of 60 s was imposed to end the
trial, which many participants reached, especially from the more remote
starting points.
After approximately 20 min of instructions and practice, participants were
tested on 18 trials, consisting of one platform-visible trial followed by two
platform-nonvisible trials, in which spatial memory was required to reach the
platform. The performance measures were the time taken to reach the platform,
the virtual distance travelled, and the number of corrections made. Only the
time to reach the platform was included in the final analysis, since the number
of corrections partly involved strategy and the virtual distance almost
perfectly correlated with, and was therefore redundant to, the time to reach
the platform. Also, participants reached the platform rapidly and with little
variation in the visible trials (a “ceiling effect”), presumably because
forward speed was set by the computer; thus, only data from the nonvisible
trials were used to assess navigational memory. 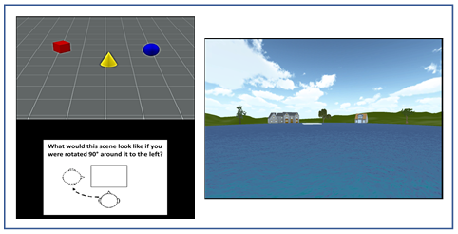
Results
Discussion
More studies with larger sample sizes are required to determine if current
widely used cognitive measures are as effective as specific topographical ones
in the earliest prediction of Alzheimers. Acknowledgements
References
Corresponding
author
Citation
Keywords