Research Article :
N Shanmugam, S Suthakaran, N Kannadasan, K Sathish kumar
In the modern scientific era the materials in the
nanoscale have gained more attention than their bulk form owing to their
peculiar physical and chemical properties. In the semiconductor industry
nanocrystalline ZnO has many applications for numerous fields, such as lasers,
sensors, solar cells and field emission devices due to its higher band gap and
larger excitation binding energy (60 MeV). Among the organic pollutants,
brilliant green (BG) creates an impact on its enriched outreaching to the
environment through effluent of textiles and paint industries [2-9]. Brilliant
green can create so many toxic effects on the human beings and animals;
therefore its degradation needs more attention. As a result of nontoxic nature
and low coast, ZnO is an alternate to TiO2 in the degradation of organic pollutants [10-12]. Doping that does
essential incorporation of ions of particular elements into the host material
to tailor its properties is a widely accepted technique in the semiconductor
industry. Further, the morphology control through doping is another major task
as the size and shape can influence various properties of the prepared
products. Doping ZnO with tellurium (Te) can tailor the luminescence properties
of ZnO by passivation of oxygen defects. Already few works are available on the
Te doped ZnO film [13-16]. Previously we have studied the photocatalytic
properties of ZnO on cerium doping. In the
present work, we have attempted to tailor the photocatalytic properties of ZnO
by incorporating Te in the lattices of Zn2+. For that tellurium in different proportions (0.1, 0.15, 0.2, and 0.25
M) are doped into ZnO through a simple chemical precipitation method. The
prepared products are analysed for their structural, optical, morphological,
and photocatalytic properties.
All the chemicals used in this study are of AR
grade with 99% purity (E.Merck) and used without further purification. Sample
preparation and dilutions were made of ultrapure water. Zinc nitrate hydrate
[Zn(NO)3.6H2O], sodium telluride [Na2Te] and potassium hydroxide (KOH) were used as precursors. For the synthesis of Te-doped
ZnO, 7.4 g (0.5 M) of zinc nitrate hydrate dissolved in 50 ml of deionized
water was stirred vigorously by magnetic stirrer and sodium telluride of
preferred mole (0.1, 0.15, 0.2, and 0.25 M) prepared in 20 ml aqueous was mixed
drop wise. Then, 6.2 g (2.5 M) of potassium hydroxide in 50 ml of deionized
water was added drop by drop to the above mixture. The entire was stirred
magnetically at 60 ºC until a white precipitate was formed. The obtained
dispersions were purified
by dialysis against de-ionized water and ethanol several times to remove
impurities. The purified products were dried in hot air oven at 100 °C for 6 h
to evaporate water and organic materials to the maximum extent. The dried
powders were pulverized to fine powders using agate mortar for further
characterizations. A similar method of preparation without the addition of
tellurium was used to synthesize undoped ZnO Nano crystals. For the purpose of studying the photocatlytic
activity of ZnO, 0.2 g of ZnO was added to a quartz photoreactor containing 100
ml of a 1 mg/l brilliant green (BG) aqueous solution. After stirring for 120
min in the dark in order to reach the absorption equilibrium, the mixture was
irradiated with sunlight with intensity fluctuation of 950 ± 25 Wm-2. The residual BG in the aqueous
solution was analyzed by checking the absorbance at 624 nm in the UV-Vis
absorption spectra. To determine the percentage of degradation of MB, the
samples were collected at regular intervals (for every 30 min), filtered and
centrifuged to remove the nanophotocatalyst particles that exist as undissolved
particles in the sample and studied using UV-Vis absorption. The degradation
percentage of the dye in the presence and absence of ZnO nanopartices can be
calculated from the following equation [18].
Where C0 is the
initial concentration of the dye and Ct is the concentration of dye after irradiation in
selected time interval. The same procedure was adopted for tellurium doped ZnO
nanoparticles. Figure 1A: X-ray diffraction patterns of ZnO and various
levels of Te doped ZnO nanosheets. Table 1: XRD derived parameters of undoped and Te doped ZnO
nanoparticles. The crystalline phase and particle size of pure and
Te-doped ZnO nanoparticles were analyzed by X-ray diffraction (XRD) measurement
which was carried out at room temperature by using XPERT-PRO diffractometer
system (scan step of 0.05° (2θ), counting time of 10.16s per data point)
equipped with a Cu tube for generating Cu K radiation (k = 1.5406 Å); as an
incident beam in the 2-theta mode over the range of 10°–80°, operated at 40 kV
and 30 mA. The photoluminescence (PL) emission spectra of the samples were
recorded with a Spectroflurometer (Jobin Yvon, FLUOROLOG–FL3-11). The
functional groups were determined by SHIMADZU-8400 Fourier-transform infrared
spectrometer in which the IR spectra were recorded by diluting the milled
powders in KBr and in the wavelength between 4000 and 400 cm-1 was used to assess the presence
of functional groups in pure and Te-doped ZnO. The morphological analysis was
performed by HITACHI S-4700 field emission scanning electron microscope
(FESEM). Energy-dispersive spectrum (EDS) analysis of the products was
performed during It has been seen that the bond
length value of ZnO increases on low level of Te doping (≤ 0.15 M) and
decreases at higher doping concentrations ( ≥ 0.15 M). The room temperature PL emission spectra of ZnO and
ZnO: Te with 320 nm excitation are shown in Figure 2. All the samples exhibit
an UV and four visible emissions. The obtained UV emission at 390 nm is
attributed to the near band edge emission of ZnO, originating from the
excitonic transitions between the electrons in the conduction bands and the
holes in the valence bands [20]. The appearance of blue emission at 437 nm
originates from zinc interstitial. The blue green emission centered at 487 nm
is due to a radiative transition of an electron from the deep donal level of Zni to an acceptor level of neutral
Vzn [21].
Figure 2: PL emission spectra of ZnO and various levels of
Te doped ZnO nanosheets. Figure 3: FT-IR spectra of ZnO and various levels of Te
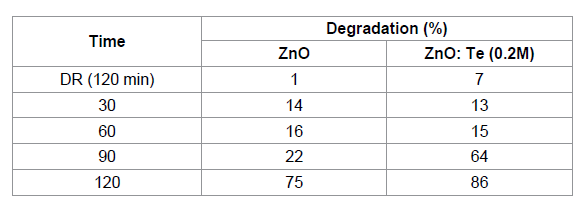
doped ZnO nanosheets. Table 2: The effect of BG dye degradation by ZnO and Te (0.2
M) doped ZnO nanosheets The intense red emission at 643 nm can be ascribed to oxygen-related
defects [22]. Significantly, the defect related emissions of the host material
can be tuned by dopant material. In the present case, the PL intensity of ZnO
strongly depends on the concentration of Te. At the initial stage, in the
absence of dopant, emission peaks have higher intensity, however, on increasing
the concentration of doping the intensity reduction can be seen with the
broadening of the FWHM as a function of Te content. This strongly suggests that
the density of oxygen defects responsible for red emissions could be controlled
by doping of Te in the host material ZnO. The same type of passivation of
oxygen defects on Te doping was reported by other researcher [15]. To know the influence of Te doping on the Zn-O
bonding, FT-IR spectra were recorded for undoped and doped ZnO in the range of
4000- 400 cm-1. The
obtained spectra are presented in Figure 3. The appearance of absorption peaks
around 3414 and 1630 cm-1 can be
ascribed to O-H stretching and bending vibrations, respectively[23]. The broad band absorbed around 2368 cm-1 can be related to the O = C = O vibration of CO2 molecule exist in air [24]. The
presence of nitrate peaks at around 883 cm-1 can be originated from zinc nitrate used as zinc source. The broad
absorption feature positioned at 431 cm-1 is due to stretching vibration of Zn-O [25]. On doping, stretching vibrations
of Zn-O are shifted to lower and higher energy regions as a result of change in
bond length. Such a change in bond length of Zn-O already has been discussed in
the XRD section.
The FESEM image of ZnO exhibits that the particles
are having spherical morphology with homogeneous size distribution (Figure 4a).
The corresponding histogram exhibits the average particle size as 23 nm (Figure
4b). The influence of Te doping on the morphology of ZnO is clearly seen from
the Figure 4c. As shown in figure, the doped product is in the form of
nanosheets stack together. These nano-sheets are showing regular hexagonal
structure of thickness 27 nm (Figure 4d). The EDS analysis of Te doped ZnO
(Figure 4e) reveals that Zn. O, and Te are the constituents of the prepared
materialIn the evaluation of photo catalytic activity of
ZnO and ZnO: Te against Brilliant green (BG), 100 ml of BG aqueous solution (1
mg/L) was taken in a quartz photo reactor. Further, 0.2 g of ZnO was added into
the quartz photo reactor. Before irradiation, the reaction mixture was stirred
in dark for 30 min to achieve the absorption- desorption equilibrium between
the catalyst and dye molecules. The Sunlight with intensity fluctuation of 950 +
25 wm-2 was used as an irradiation
source. During the course of light irradiation, 5 ml of sample was collected at
regular intervals (every15 min), filtered and centrifuged to remove the
undissolved photocatalyst. The filtrate was analyzed by UV-Vis spectrometer at
Pmax = 624 nm and the photodegradations were calculated by equation (1). The photocatalytic activity is based on the
reactive nature of an electron-hole pair generated in the semiconductor
nanoparticles. Under illumination by light of energy greater than the
semiconductor band gap, electron is excited to the conduction band and electron
in the conduction band migrates to the lattice surface. If no recombination
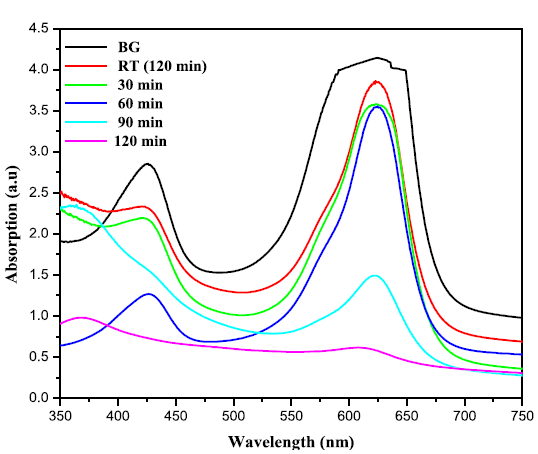
takes place, these charge carriers can react with adsorbed molecules. Figures 5(a) and 5(b) exhibit the change in
absorbtion pattern of BG exposed to Sunlight for varies irradiation times in
the presence of ZnO and ZnO: Te, respectively. The percentage of degradation
was calculated using the equation 1 and the results are given in Table 2. From
the table, it is clear that after 120 min of light irradiation, 75 % of dye was
degraded in the presence of ZnO. However, for ZnO: Te, the degradation rate was
increased to 86% at the same 120 min of light irradiation. This result revealed
that Te doped ZnO exhibited higher photocatalytic activity than pure ZnO. Further,
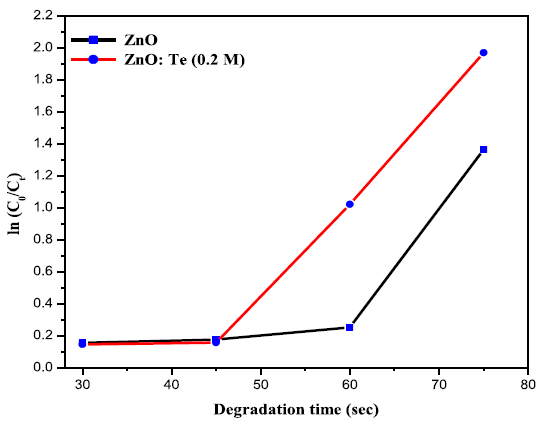
the rate constant values for dye degradation for the catalysts were calculated
using the first order rate eqution [17].the samples and from the slope of the graph, rate constant values were
calculated. The value of rate constant for undoped ZnO was found to be 0.01235
min-1, whereas, for ZnO: Te, it was
increased to 0.02111 min-1. The
increased k value suggests the improved photocatalytic activity of ZnO on Te
doping. The possible mechanism behind the improved photocatalytic activity of
Te doped ZnO is explained as follows. When light of energy greater than forbidden gap is
irradiated, electrons from the valance band can make a quantum jump to
conduction band of ZnO. The presence of dopant Te4+ within the crystal matrix or on the surface of ZnO can trap the
photogenerated electrons or holes and subsequently transfer the same to
adsorbed oxygen and hydroxyl ions to generate super oxide radicals (O2•ˉ) and hydroxide radicals (•OH), respectively. This behavior
will reduce the electron-hole recombination and generates more and more free
radicals responsible for the degradation of BG. In this way ZnO doped with Te
reveals higher activity to the degradation of the dye brilliant green. In summary, high quality nanocrystals of pure and
Te doped ZnO have been synthesized through a simple chemical precipitation
approach. The XRD patterns suggest the formation of wurtzite ZnO nanocrystals
with a size of 24 nm. The sizes of the samples could be controlled up to the
concentration of 0.2 M of tellurium and were found to be independent at 0.25 M
of doping. The PL spectra of the doped products show the intensity quenched red
emissions suggesting the passivation of oxygen defects. The change in the ZnO
bond length on doping has been confirmed by FT-IR analysis. The FESEM analysis
of the products reveals a change in morphology of ZnO from spherical particles
to nanosheets on Te doping. The EDS pattern of Te-doped ZnO exhibits the presence
of Zn, O, and Te as expected. The doping of Te showed an enhancement of the
photocatalytic activity of ZnO against brilliant green (BG). The enhanced
photocatalytic activity of Te doped ZnO suggesting its usage as a scavenger
against the pollutant brilliant green which is being discharged from the
textiles industries.
Department of Physics, Annamalai University, Chidambaram 608 002, Tamilnadu, India.Tel: +91-9444276357 E-mail: quantumgosh@rediffmail.com. Shanmugam N, SuthakaranS, Kannadasan N, Sathishkumar K
(2015) Synthesis and Characterization of Te Doped ZnO Nanosheets For
Photocatalytic Application. J O Heterocyclics 105: 15-20 diffraction, photoluminescence, degradation,
brilliant green,photocatalyticSynthesis and Characterization of Te Doped ZnO Nanosheets For Photocatalytic Application
Abstract
Full-Text
Introduction
Materials and Methods
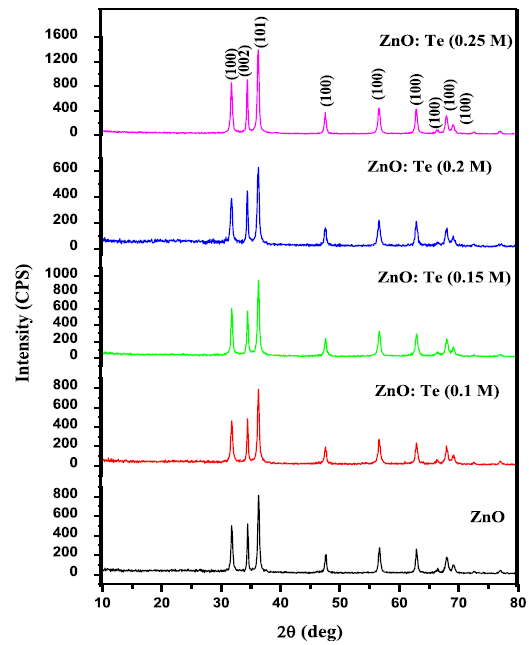


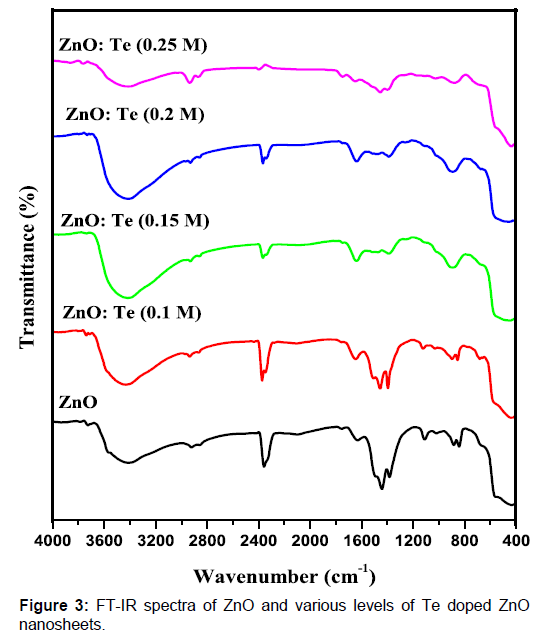
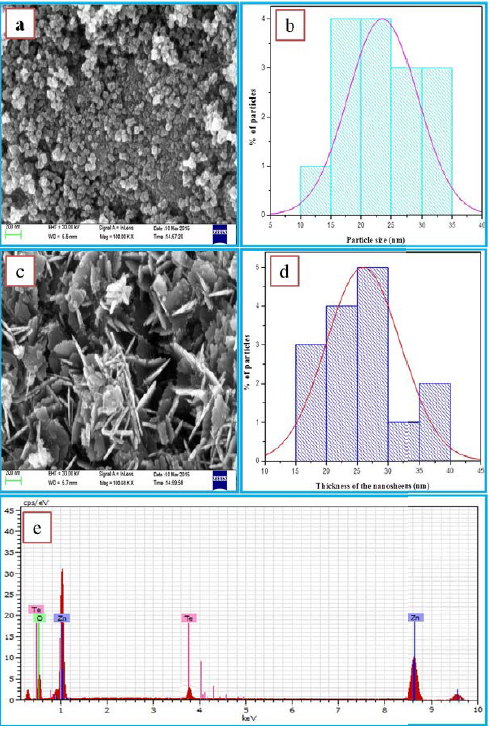
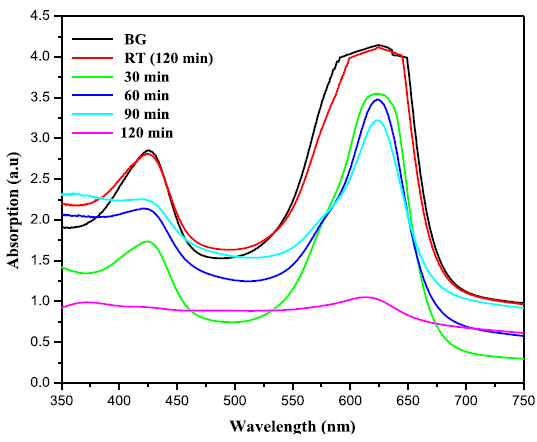
Figure 5A: Time dependent UV-Vis absorption spectra of the photo
catalytic degradation of BG in the presence of ZnO nanoparticles.
Conclusion
References
2. Huang MH, Mao S, Feick H, Yan H, Wu Y, et al. Room-temperature ultraviolet nanowire nanolasers (2001) Science 292:1897-1899.
3. DM Bagnall, YF Chen, Z Zhu, T Yao, S Koyama, et al. Optically pumped lasing of ZnO at room temperature (1997) Appl Phys Lett 70:2230-2232.
4. ZS Wang, CH Huang, YY Huang, YJ Hou, PH Xie, et al. A highly efficient solar cell made from a dye-modified ZnO-covered TiO2 nanoporous electrode (2001) Chem Mater 13:678.
5. Z Liu, C Liu, J Ya, E Lei. Controlled synthesis of ZnO and TiO2 nanotubes by chemical method and their application in dye-sensitized solar cells (2011) Renew. Energy 36:1177.
6. CJ Lee, TJ Lee, SC Lyu, Y Zhang, H Ruh, et al. Field emission from well-aligned zinc oxide nanowires grown at low temperature (2002) Appl. Phys. Lett 81:3648.
7. Law M, Greene LE, Johnson JC, Saykally R, Yang P. Nanowire dye-sensitized solar cells (2005) Nat Mater 4:455-459.
8. D Sridevi, KV Rajendran. Synthesis and optical characteristics of ZnO nanocrystals (2009) Bull. Mater. Sci 32:165.
9. Goldberger J, Sirbuly DJ, Law M, Yang P. ZnO nanowire transistors (2005) J Phys Chem B 109:9-14.
10. YH Jang, ST Kochuveedu, MA Cha, YJ Jang, JY Lee, et al. Synthesis and Photocatalytic Properties of Hierarchical Metal Nanoparticles/ZnO Thin Films Hetero Nanostructures Assisted by Diblock Copolymer Inverse Micellar Nanotemplates (2010) J. Colloid Interface Sci. 345:125-130.
11. M Wu, B Yang, Y Lv, Z Fu, J Xu, et al. Efficient one-pot Synthesis of Ag Nanoparticles Loaded on N-Doped Multiphase TiO2 Hollow Nanorod Arrays With Enhanced Photocatalytic Activity (2010) Appl. Surf. Sci, 256:7125-7130.
12. MR Hoffmann, ST Martin, W Choi, DW Bahnemann. Environmental Applications of Semiconductor Photocatalysis (1995) Chem. Rev 95:69-96.
13. Savas Sönmezoglu, Erdi Akman. Improvement of physical properties of ZnO thin films by tellurium doping (2014) Applied Surface Science 318:319-323.
14. A Iribarren, P Fernández, J Piqueras. Recombination processes in Te-doped ZnO microstructures (2013) Phys. Status Solidi B:1–6.
15. Farid Jamali Sheini, Ramin Yousefi, MR Mahmoudian, Nabeel Ali Bakr, Abdolhossein Sa, et al. Facile synthesis of different morphologies of Te-doped ZnO nanostructures (2014) Ceramics International 40:7737-7743.
16. A Iribarren, P Ferna´ndez, J Piqueras. Cathodoluminescence study of Te-doped ZnO microstructures grown by a vapour–solid process (2008) J Mater Sci 43:2844–2848.
17. N Kannadasan, N Shanmugam, S Cholan, K Sathishkumar, G Viruthagiri,et
al. The effect of Ce4+ incorporation on structural, morphological and photocatalytic characters of ZnO nanoparticles (2014) Materials Characterization 97:37-46.
Pouretedal HR, Norozi A, Keshavarz MH, Semnani A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes (2009) J Hazard Mater 162:674-681.
19. K Sathishkumar, N Shanmugam. N Kannadasan, S Cholan, G Viruthagiri. Influence of Zn2+ ions incorporation on the magnetic and pseudo capacitance behaviours of NiO nanoparticles (2014) Material Science in Semiconductor Processing 27:846-853.
20. N Kannadasan, N Shanmugam, S Cholan, K Sathishkumar, R Poonguzhali,et al. Synergistic effect of bimetal ions (Ce, Pb) incorporation on optical, structural, and sensory activity of ZnO nanocrystals (2014) J Solid State Electrochem 3:757-768.
21. Gunjan Srinet, Ravindra Kumar, Vivek Sajal. Structural, optical, vibrational, and magnetic properties of sol-gel derived Ni doped ZnO nanoparticles (2013) Journal of Applied Physics 114:033912.
22. OL Stroyuk, VM Dzhagan, VV Shvalagin, SY Kuchmiy. Size-Dependent Optical Properties of Colloidal ZnO Nanoparticles Charged by Photoexcitation (2010) J. Phys. Chem. C 114:220.
23. K Sathishkumar, N Shanmugam, N Kannadasan, S Cholan, G Viruthagiri. Opto, magnetic and electrochemical characterization of Ni1-xCoxO nanocrystals (2014) J. Mater Sci: Mater Electron 3:1881-1889.
24. Shi L, Gunasekaran S. Preparation of Pectin-ZnO Nanocomposite (2008) Nanoscale Res Lett 3: 491-495.
25. N Kannadasan, N Shanmugam, K Sathishkumar, S Cholan, G Viruthagiri, et al. Optical behavior and sensor activity of Pb ions incorporated ZnO nanocrystals (2015) spectrochimica Acta Part A: Molecular and Biomolecular spectroscopy 143:179-186. *Corresponding author:
Citation:
Keywords