Research Article :
Mohamed A. El Hamd , Ahmed A. H. Abdellatif*
A
sensitive, selective, and validated spectrofluorimetric method is being
described for the determination of penicillin G. The method utilizes this drug
(as a source of the amine moiety) to react with acetylacetone and formaldehyde
through the Hantzsch reaction. The faint yellow product of dihydropyridine
derivatives was measured at an emission wavelength of 481 nm (after excitation
at 416 nm). Linear calibration graphs were obtained in the range of 0.2-200 μg/mL.
The experimental limits of detection and quantitation were 0.170 and 0.515 μ/mL,
respectively. The relative standard deviation (n=5) at the 100 μg/mL level were
6.11 for intra-day precision and 9.42 for inter-day precision. The method was
successfully applied in the analysis of pharmaceutical formulation the recovery
was quantitative, and the result obtained agreed with those obtained by another
reported method. Penicillin
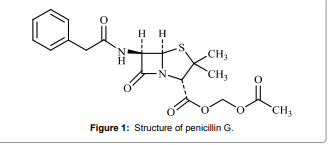
(Figure 1) is
considered a broad-spectrum antibiotic. It is referred to as a β-lactam antibiotic,which are among the
oldest and the most valuable clinical antimicrobial agents used to treat the
bacterial infections of skin, soft tissues, and urinary tract [1]. Due to the
presence of penicillin on the Egyptian Market until now, and Egyptian
clinicians still use this drug to treat many diseases, we optimized a new
method for the easy identification of penicillin. The optimized method was applicable,
reliable, and economic. In this study, the advantages of fluorimetric analysis (availability
in all quality control laboratories, simplicity, high precision, rapidity, and low
reagents and solvents consumption) are combined with the benefits of utilizing the
Hantzsch condensation reaction for the spectrofluorimetric determination of penicillin.
The chemical reaction variables were studied and the fluorescence variables were
optimized on the basis of sensitivity, temperature, and reagent consumption. Figure 1: Structure of penicillin G. Apparatus All
spectrofluorometric measurements were performed by using a SCINCO spectrafluorimeter
(Scinco FS-2, Korea) with a matched 1 cm thick quartz cell. A computer loaded with
application software (FluroMaster Plus, version 4.2 Bul, dO3), pH meter
(Milwaukee MW101, Atlanta, Georgia, US), andthermostatically controlled water
bath (Memmert GmbH, Schwabach, Germany) were used. Material All
chemicals were of analytical grade and were used without further purification. Bi-distilled
water was used throughout. Reference material penicillin was kindly provided by
CID-Developing Chemical Industry Co., Egypt. The procedure was applied on vial
formulation which was purchased from the local market. Penicillin solutions: Stock
solutions of penicillin (1 mg/mL) were prepared in bi-distilled water and then
the working solutions were prepared by further dilution with bi-distilled water
to cover their linearity range. Formaldehyde and acetyl acetone solutions: Formaldehyde,
(20% v/v) and acetylacetone (10% v/v) (El-Nasr Chemical Co., Egypt) solutions
were freshly prepared by mixing 58.8 and 10.20 mL respectively, to 100 mL bi-
distilled water. Preparation of pharmaceutical dosage form samples: Three
vials were weighed and mixed thoroughly, a weighed amount of the powder
equivalent to 25 mg of drug was dissolved in 25 mL bidistilled water, mixed
well and filtered. Further dilutions with the same solvent were made. 1.0 mL
of sample or standard solution was transferred into a 10-mL calibrated flask.
1.0 mL of acetylacetone and formaldehyde (in the same order) were added, mixed
well, and allowed to stand for 50 min in a water bath previously heated to 100OC.
After cooling, the solution was completed to the mark with bi-distilled water
and measured at the emission wavelength (Em) of 481 nm, after excitation at an
excitation wavelength (Ex) of 416 nm. The
investigated penicillin drug exhibits very low native fluorescence intensity
therefore a derivatization reaction is required to improve its sensitivity and
also the selectivity away from the other ingredients associated with its dosage
form. The investigated penicillin contains an amine, which is known to react
with acetylacetone and formaldehyde through the Hantzsch reaction.
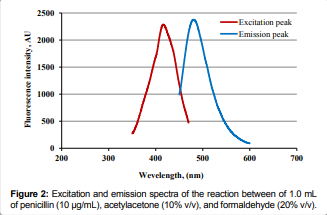
Moreover, the reaction product exhibited strong, reproducible fluorescence at
an Em of 481 nm. [Figure 2]. The
Hantzsch reaction is a known condensation reaction that was reported
in the literature as a useful pathway for pyrrole and pyridine synthesis. In
the same manner, a combination of acetylacetone and formaldehyde can react with
a source of amines forming a faint yellow
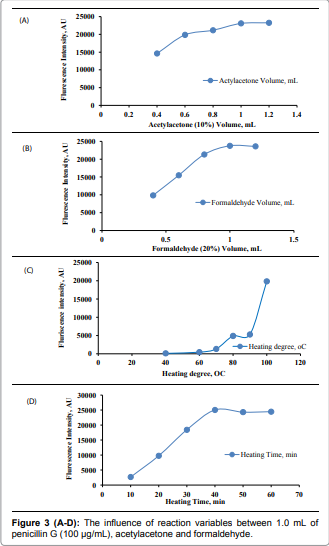
product that can be measured spectrofluorimetrically [2]. Effect of reagents concentration: One
mL of 10% v/v acetylacetone and 20% v/v formaldehyde were the most suitable concentrations
which gave the maximum fluorescence intensity for
the proposed method. [Figure 3 (A) and
(B)]. Effect of heating degree and time: Heating
at 100OC for 50minutes
was sufficient to produce maximum fluorescence intensity the produced faint
yellow colors were stable for more than one hour, which made it suitable for
multiple sample measurements. [Figure 3
(C) and (D)]. Effect of diluting solvent: Different
diluting solvents were tried such as water, ethanol, methanol, acetonitrile,
and acetone but the most stable fluorescence intensity was obtained using water
as the solvent for the reaction. Content uniformity: The content
uniformity test was carried out by randomly testing for the selected dosage
forms asdescribed previously [3]. The content uniformity of all the vials had
international Pharmacopeia (IP) limits of 85 - 115% of drug and none contained
below 75 or above 125% of drug as shown in Table
3. The
methods were tested for linearity, accuracy, precision, and linear regression
equations were obtained. The linearity equation is represented by Y = a + bX,
where Y is fluorescence intensity,
X is the analyte concentration, and a and b are the slope and intercept
respectively. The regression plots showed a linear dependence on the absorbance
over the concentration range given in Table 1. The
table also shows the results of the statistical analysis of the experimental
data, such as the slopes, the intercepts, and the correlation coefficients (r)
obtained by the linear least-squares treatment of the results. The limits of
detection (LOD) and the limits of quantitation (LOQ) were determined by
establishing the lowest concentration that can be measured according to the
international conference of harmonization (ICH) recommendation [4]. The LODs
and LOQs were calculated according to the following equations: LOD = 3.3 Sa/b
and LOQ = 10 Sa/b where Sa
is the standard deviation of the blank, and b is the slope of
the regression line, the results are shown in Table
1. Table 1: Quantitative parameters for analysis of penicillin G by the proposed
method. Accuracy and precision: Intra- and
inter-day assay precision and accuracy were assessed using five replicate
measurements at one concentration level [5]. Results of recovery studies with pure
penicillin G by the proposed method show a small value of standard deviation
and variance that indicates low scattering of the points around the calibration
line and high precision as shown in Table 2. Table 2: Accuracy and precision analysis of the proposed method at oneconcentration level. Finally,
the proposed method was successfully applied to determine penicillin G in
multiple vials. The results obtained were statistically compared to those
obtained for the reported method [6] by the studentst-test for accuracy and the variance ratio
F-test for precision as recorded in Table 3.
The experimental values of t and
F did not exceed the theoretical values, indicating a lack of significant
difference between the compared methods. Based on the data in Table 3, it was found that the amino moiety over
all demonstrated better results in the proposed method compared to those of the
reference methods concerning the values of the SD. This may be attributed to
the greater nucleophilicity of the amino moieties therefore, the reaction in
the proposed method goes more smoothly resulting in better reproducibility and
hence better values of standard deviations. The
proposed method is economic and selective for the determination of the
investigated penicillin G in bulk and in the marketed form. There is no
requirement for any sophisticated apparatus
as in chromatographic methods. Omission of an extraction step with organic
solvents is an added advantage. The method has been validated in terms of its
reproducibility, precision, and accuracy suggesting its suitability for the
routine analysis. 1. Sweetman
S, Martindale: The Complete Drug Reference (2009) The Pharmaceutical Press,
(36th ed.,) London. 6. Levy G.B, Shaw D, Parkinson E.S, Fergus D. Determination of
penicillin G,a spectrophotometric method, Analytical Chemistry (1948) 20:
1159-1161.Hantzsch Reaction as a Method for Spectrofluorimetric Analysis of Penicillin G
Abstract
Full-Text
Introduction
Experimental
Reagents and Solution
General Recommended Procedure
Results and Discussion
Influence
of reaction variables
Validation
of the proposed method
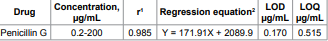

Application
of the proposed method to analyze the dosage forms

Conclusion
References
2. Hanaa
M. Saleh, Magda M. EL-Henawee, Gamal H. Ragab, Omnia F. Mohamed,
Spectrophotometric and spectrofluorimetric determination of pregabalin via condensation
reactions in pure form and in capsules (2014) International Journal of
Pharmaceutical, Chemical and Biological Sciences 4 :738-747.
3.
Katori N, Aoyagi N, Kojima S. The study of the applicability of content uniformity
and weight variation test--the state of commercial tablets and capsules in
Japan (2001) Chem Pharm Bull (Tokyo) 49: 1412-1419.
4. ICH-
Q2 (R1) "International Conference on Harmonization of technical requirements
for registration of pharmaceuticals for human use, Text and Methodology"(2005).
5.
Washington D. C. The United States Pharmacopeia 31 and NF 26. The National
Formulary, American Pharmaceutical Association (2008). Keywords