Research Article :
Waseem Ullah Shah, Dil Faraz khan, Shahzeb Burki, Mohib Ullah Khan and Haiqing Yin This research article reports simulations and
prediction based calculation for Co-Fe system under the application of Calphad
method and thermo-calc package. At different elevated temperature of 1200k,
1225k 1nd 1250k the said system is modeled and corresponding behavior of Gibbs
free energy, phase diagram and activity curve is monitored. As per calculation
the Gibbs energy curve is correspond to its negative era which shows the actual
stability application of the said alloy system. The alloying element shows
strong interaction amongst which results negative deviation from Roults law in
activity era. At 1250k the activity value becomes maximum with same negative
deviation. This shows the applicability and reliability of the said alloy
system. The
modeling and simulation study is the worst need of ongoing technologies as per
the costly slandered and difficult pattern of experimental era. As by modeling
we can reduce the time and cost with precise calculations of thermodynamic
parameters gradually as calculated by Gibbs for thermodynamics and phase based
equilibria. In 1929 an Elman predicted the magnetic sense of alloys
Fe-Co system through high Curie
temperature and predicted its reportable permeability and saturation most
magnetizations [1,2]. The
thermodynamic parameters of the said iron-cobalt system have been studied well
by those electrical engines based on magnetic properties and materials has
results best prediction of this field as discussed by the software and flexible
tool thermo cal is used for various thermodynamic calculations and predictions
and also helpful by solving and interaction among various elements with many
ideal and non-ideal based behaviors .1n 1981 the first version of this package
was installed and in 2002 their modern and many database version is established
with various thermo dynamical calculation capacity with description in 1985 by Erwin
povoden, et al. [3-8]. It
is the calculation of phase
diagrams method which is useful for finding thermodynamic parameters of
many materials with prescribed phase diagrams and phase equilibria, it has many
modules for calculations and simulations, recently it has been updated for many
thermodynamic properties of different materials in form of molar volume,
thermodynamic elastic moduli, and phase like properties, their range of
validity is also applicable for processing elements verses composition molar
and molar temperature verses Activity with thermodynamic and optical
properties, all that calculation is done by calculation of phase diagram method
[9-12]. Figure
1:
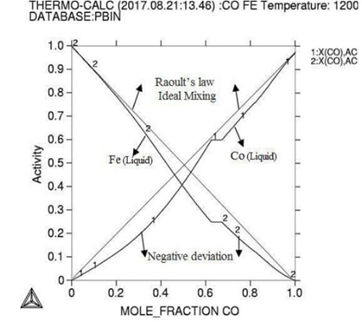
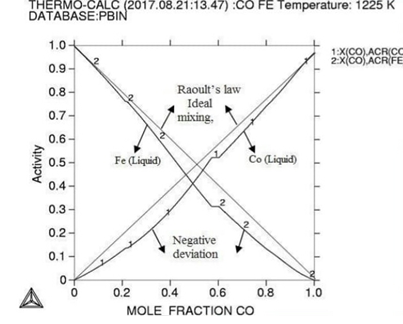
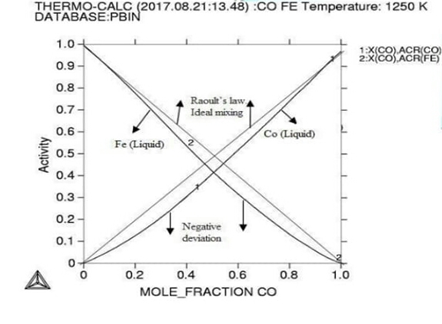
Molar activity curves at respective 1200K, 1225K, and 1250K temperature. Figure
2:
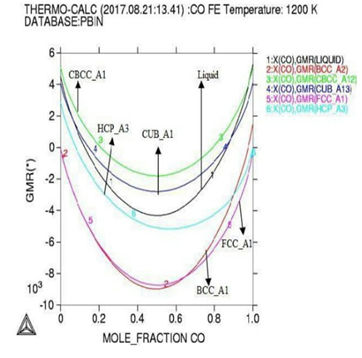
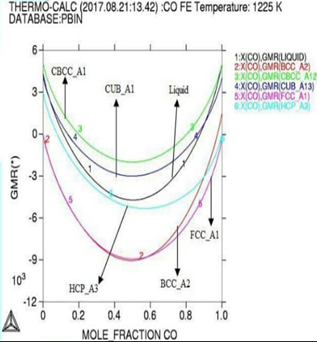
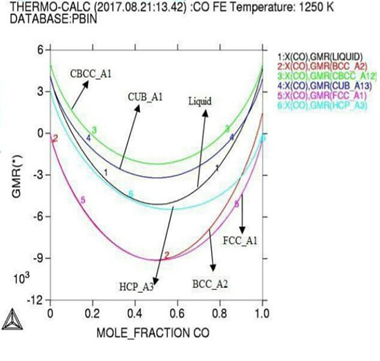
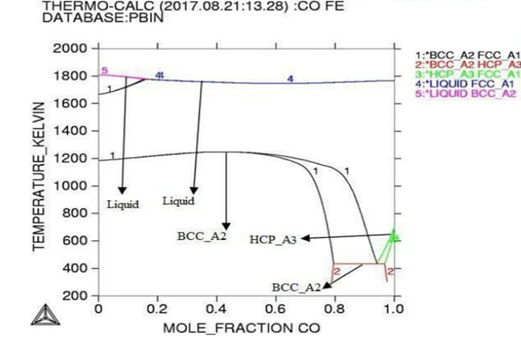
Gibbs energy curves at respective 1200K, 1225K, and 1250K temperature. Figure 3: Phase diagram of the Co-Fe binary alloy system. In
this research paper the thermodynamic calculations with valid predictions
through PBINE database is employed at various temperatures of 1200K, 1225K,
1250K, the activity behaviors is investigated with phase equilibrium and all
module used with lowering occurs in Gibbs
energy curve as shown by (Figures 1
and 2). Here in calculation,
the activity is found increasing as with temperature and all the deviation here
is accordance with Roults
law ideal curve [13]. As concern with calculated phase
diagrams it is in accordance with literatures. Thermodynamic analysis of Co-Fe
system is carried out by getting better description for five stable phases
founded in Co-Fe
binary system. The four among them hcp,fcc,bcc and liquid ate founded the
stable phases in Co-Fe system by considering substitution solution phases, the
molar Gibbs energies of the particular phases are being calculated by
sub-regular solution and substitution model. As their Gibbs energy is analyzed
as according to Inden, et al. and modified by k.oikawa, et al. [13-15]. Where
in Gibbs energy model, oGcr and oGfe are Fe, Co pure phases in solution, as xi
denotes molar fraction of elements in solution, as Fe and Co mole fraction in
solution, while the excess energy is shown by constant parameter β ij. Gm is
the magnetic part of Gibbs energy. As
by temperature dependent section, we have Where
mL ij, are the excess binary energies
of binary mixing system. While a, b, c, d, e, f are model parameters. The
magnetic part of Gibbs energy is given as as
Q ≤ 1, k(α) is normalized state polynomial of temperature, as by bohr magneton
number. k(α) = -[α-5/10+/497+α-15/315+α-25/1500]/Q while
Q > 1, Q = (11692-11692p/15975p)+518/1125 then
k(α) = -[α-5/10+/497+α-15/315+α-25/1500]/(11692-11692p/15975p)+518/1125 As
p is constant, whose value varies for different phases, for bcc is 0.4 and for
other phases is 0.28 [16]. phase
are of activity based on highest strong atomic interaction based, FCC_A1=1
phase is found attached with highest temperature that indicated austenite solid
solution for Cobalt-Iron
system, all the phases calculated here are at different temperatures and
molar compositions are in well accordance with data in literature, in between
(400-450)K the magnetic properties occurs in alloy as ferromagnetic
era at the concentration of (0.85-1.0%), that region is important for
magnetic field industry. As
our results indicating that at highest temperature range of 1250K, and
concentration of (0.4-0.6%) the Gibbs energy goes to its lowest value which
shows that the constituent particle is having strong negative interactions and
the alloy is reporting best stable behaviors at that temperature although the
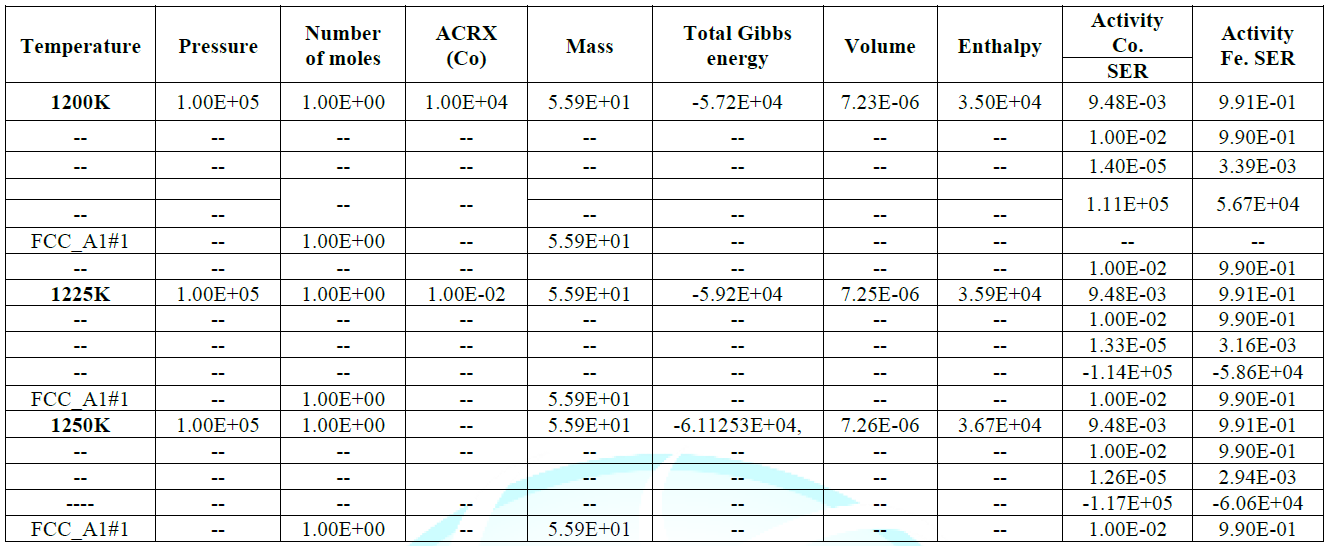
activity value is also at peak as confirmed from table during calculations,
different thermodynamic parameter like volume, enthalpy and entropy verses
temperature is varied here for confine result. At 1250k the smooth negative
deviation is seen from ideality at the range of 0.25-0.65 of molar composition
of alloying elements, the stage indicate here an ideal mixing and solid
solution and well good epitaxial growth of the alloying elements.Then
lattice elements and brillouin zones have no lack matching of alloy elements at
1250k. at range 0.8-1.0 of composition the well good magnetic properties in said
alloy
system are having importance for industrial sectors. The table indicates
frequent increase in the enthalpy
values for increasing heat contents of the alloying elements with
increasing temperature up to 1250k shown highest enthalpy of 3.67311E+04 with
corresponding Gibbs energy of -6.11253E+04, that indicate the vital stability
of the said alloy system at high temperature ranges. Here
the thermodynamic calculations and predictions of binary alloy Co-Fe system has
been studied using Calphad approach. Thermodynamic analysis involves
calculation of phase diagram, Gibbs energy of mixing, excess Gibbs energies,
thermodynamic molar activities, and coefficient of activities, partial and
integral values of enthalpy for Ag-Cu alloy system at three elevated
temperatures 1200K, 1225K, and 1250K. The alloy reports positive deviation from
Vegards
law and corresponding good negative deviation from Rout’s law ideal Gibbs
curve. Waseem
Ullah Shah, Department of physics, University of science and technology Bannu
28100, kpk, Pakistan, E mail: waseemullahshah303@gmail.com Shah WU, Khan DF, Burki
S, Khan MU and Yin H. Modeling, simulations, predictions, calculations and
thermodynamic assessments of cobalt-ferric binary alloys system using calphad
method and pbine database (2020) J of Heterocyclics 2: 3-6. (Co-Fe) system, Calphad, Thermo-calc, Thermodynamic
calculations.Modeling, Simulations, Predictions, Calculations and Thermodynamic Assessments of Cobalt-Ferric Binary Alloys System Using Calphad Method and Pbine Database
Abstract
Full-Text
Introduction
Calphad Method
Results and Discussion
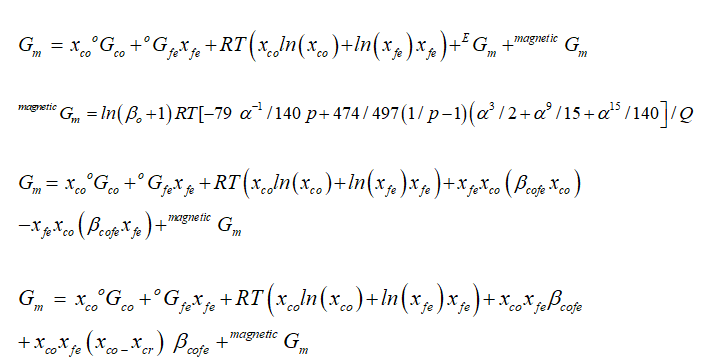
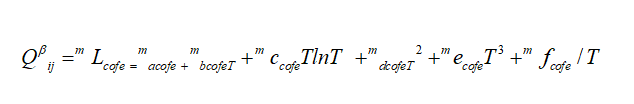
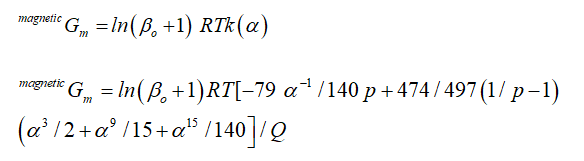
Conclusion
References
Corresponding
author
Citation
Keywords