Research Article :
Ferruh Aşçi, Mehmet İnak and Sait Bulut This study was carried out with Hydrachna processifera
(Acari, Hydracnidia), Eylais setosa and Hydrodroma despiciens common in lakes.
Fatty acid ratios were evaluated comparatively in terms of species. For this
purpose, these species collected from the Karamık lake (Afyonkarahisar-Turkey)
were analyzed by GC-MS gas chromatography in the laboratory. In the results of
the analysis, saturated fatty acids such as myristic (C14:0), palmitic (C16:0),
heptadecanoic (C17:0), and stearic acid (C18:0) were observed in high amount.
There was a significant difference between species in terms of total saturated
fatty acid ratios. The monounsaturated fatty acids palmitoleic (C16:1), oleic
(C18: 1), and erucic (C22:1) are the most common acids. The major
polyunsaturated fatty acids were linolenic (C18:3), eicosatrienoic (C20:3),
eicosapentaenoic (C20:5) and docosahexaenoic (C22:6) acid, linoleic (C18:2),
gamma-linolenic 3), eicosadienoic (C20:2) and arachidonic (C20:4) acids. At the
end of the study, there were considerable differences between the species
studied in terms of monounsaturated and polyunsaturated fatty acids in these
water mite species. This study also found that the fatty acid composition was
different for each species and this is important for the molecular taxonomy of
water mites. Water mites are one of the polyphilic groups in the Acari
subclass. They are known as Hydracarina, Hydrachnidia or Hydrachnellae. Over
6.000 species have been defined worldwide, representing 57 families, 81
subfamilies and more than 400 genera. Water mites have a complex life cycle.
Their eggs are found on many different water plants in the water. They live in
different animal species as ectoparasites in larval stages [1-3]. There is a
special significance in the determination of living areas and communities in
streams, lakes and ponds. The water mites which spread almost all over inland
waters are used as biological indicator organisms in the determination of clean
water resources [3-8]. Up to this time, it seems that the studies on the water
mites are classical systematic studies. Recently, ecological, genetic, and
other molecular studies have also been carried out in this group [9-16]. The
determination of fatty acid compositions has been done in water mites
(Hydrachnadia) group, for the first time in the present study. In this study,
the fatty acid ratios of water mites species were determined and similarities
between species have been discussed in terms of these ratios. Oils are one of
the important organic compounds required for all living things, including
humans. In addition to being a high energy source, they are very important in
terms of combining with proteins to form lipoproteins and to contain fat
soluble vitamins [17,18]. Fatty acids are monobasic organic acids with a straight
chain and varying chain length, usually containing a double number of carbon
atoms. All fatty acids have long hydrocarbon chains, a methyl group at one end
of the chain and a carboxyl group at the other end. The presence of long or
short chain fatty acids depends on the number of carbon atoms it contains and
ranges from 4 to 26. Fatty acids predominantly present in fats usually contain
16-18 carbon atoms. The fatty acids to which all of the carbon atoms are bound
by a single bond are called saturated fatty acids (e.g., palmitic acid). Fatty
acids containing at least one double bond between carbon atoms are called
unsaturated fatty acids (e.g., oleic acid). If there is more than one double
bond between carbon atoms, it is called polyunsaturated fatty acid (e.g.,
linoleic acid) [19]. According to their physical properties, unsaturated fatty
acids up to 10°C are present in liquid form at room temperature, while longer
chain fatty acids are solid [20]. Studies on fatty acids have been done more in
vertebrates, but much less in invertebrate species. In the Acari team, which is
an invertebrate subgroup, these studies are seen only in terrestrial forms
[21-24]. The fatty acids contained in some Acari species have been
identified in the study entitled: “Variability in cuticular hydrocarbons and
phenotypic discrimination of Ixodes ricinus populations (Acarina: Ixodidae)
from Europe” by Estrada-Pena and coworkers and in the study “Fatty acids as
cuticular surface components in oribatid mites (Acari: Oribatida)” by Raspotnig
and Krisper [25] and in the study “Cuticular fatyy acid profile analysis of
three Rhipicephalus tick species (Acari: Ixodidae)” by Shimshoni et al. [26]. No studies have been done about water mites on this subject.
This study has been the first on this subject. In this study, it has been seen
that the similarities in fatty acid compositions increase with the systematic
proximity of water mites. Collection of samples Determination of fatty
acids Chloroform: Methanol mixture (2:1) was used to obtain oil
samples [27]. To obtain crude oil, chloroform:methanol (2:1). 30 ml mixture was
added to 0.1 g of the fractionated water mites samples. The samples were then
crushed until they became slurry with 24000 rpm ultra-homogenizer. The mixture
was filtered with filter paper (1st filtration). The residue on the filter
paper was removed and chloroform: methanol mixture (20 ml) was added and
homogenized for a second time. The resulting slurry mixture was again filtered.
The first and second filtrates were combined. It was then taken up in a 250 ml
separating funnel and 20 ml of reagent solution was added and shaken well and
the phases were allowed to stand until separated. The organic phase (chloroform)
was taken up in the evaporation flask and the solvent was completely evaporated
in the Heidolph-2 brand vacuum rotary evaporator at 45ºC. Chloroform remaining
in the oil was removed with dry nitrogen and crude oil was obtained.Esterification
process: A 16-20 mg homogenized oil sample was taken in a capped cap and 4 ml
of 2% methanolic NaOH solution was added. The tube was sealed with nitrogen gas
and then boiled for 10 minutes until saponification occurred on the water bath.
At the end of the saponification, 2 ml of 14% BF3-methanol complex was added to
the cooled mixture and boiled for more 5 min. Then the tube was cooled to 30-40ºC and shaken vigorously
for 30 seconds by adding of isooctane. 4 mL saturated NaCl solution was added
over it. After the mixture was thoroughly shaken, it was taken into the
separation funnel and allowed to stand for 5 to 10 minutes to separate the
phases. At the end of the extractions, the upper phase (organic phase) was
taken and dried with Na2SO4.It was then passed through special filters of 0.45
mm in diameter and placed in vials and filled with nitrogen gas to close the
caps tightly [28]. This extract was injected into gas chromatography [29].Injecting
the samples into the gas chromatograph: Fatty acid methyl esters were analyzed
by gas chromatography (HP Agilent 7890A) using an HP capillary column [(100-m
length and 0.25-mm internal diameter and 0.20 μm of film thickness; HP 88)]. • Injector
temperature: 250°C, detector temperature: 250°C, carrier gas: H2, 30 mL / min. • Split
ratio 50:1, split, flow rate 71.0 ml/min. temperature program, initial 80°C for
50 minutes, temperature 10°C/minute for 40 min at 210°C. The methylated extract was taken with an automatic injector
of gas chromatography and peaks were detected in the chromatograph. The peaks
obtained from the samples were identified by comparing the fatty acids with the
standard peaks and fatty acids were calculated as percentages.Fatty acid methyl
ester standards: To determine the fatty acids, mix standards containing 37
fatty acids and mix standards containing 4 and 5 fatty acids were used. The
fatty acids in the samples were determined by comparing the peaks of the
samples with the peaks of the fatty acid standards. Peaks of fatty acids in the
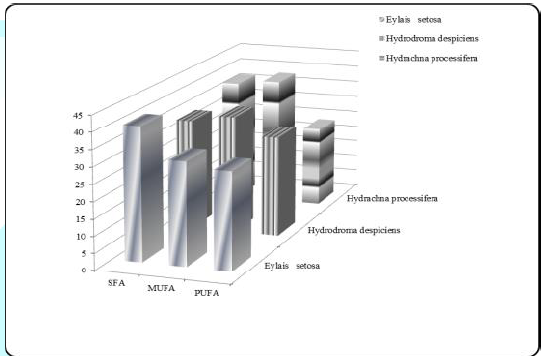
standard are shown in Figure 1. Figure
1: Average SFA, MUFA and PUFA ratios (%) for
species living in Karamık Lake. Statistical evaluation: Statistical analyses were performed
with the SPSS 18.0 computer program. First, the normality test of the data was
performed. Single and two-way analysis of variance was used because the data
showed normal distribution and there were more than two groups. The Turkey test
was used for multiple comparison tests of homogeneous groups and the Tamhane test
was used for the non-homogenous groups by controlling the homogeneity of the
variances. Also, the arithmetic average ± standard deviation values were given
for each group. This study was carried out with common species of water mites
(Acari, Hydrachnidia) Eylais setosa, Hydrodroma despiciens, and Hydrachna
processifera in lakes. These three species are included in a separate water
mite family group. In the present study, fatty acid compositions were
determined for the first time in water mites species.No studies have been found
on the identification of fatty acid compositions in the water mites until now.
However, few studies have been made on this issue with some terrestrial and
parasitic species of Acari. Among these, some notable studies are as follows:In
their study, Aboshi et al. have reported that Tyrophagus similis and Tyrophagus
putrescentiae (Astigmata: Acaridae) species have the ability to biosynthesize
linoleic acid [(9Z, 12Z) -9, 12-octadecadienoic acid]. Murungi and colleagues
reported that fatty acids in essential oils (camphor, limonene, decanoic acid,
hexadecanoic acid, dodecanoic acid) from the leaves and fruits of Solanum
sarrachoides plant had negative effects on the laying of Tetranychus evansi
(tomato spider mite) [30]. In another study, Maazouzi et al. investigated the effects
of diarrhea and feeding on the hepatopancreatic fatty acid composition of the
Eriocheir sinensis species at different periods and they reported that total
saturated and monounsaturated fatty acids differed by nutritional status [31].In
a similar study, Wen et al., showed that fatty acid analysis of phospholipids,
neutral lipids and total lipids of the Unio elongatulus species showed the
highest C16:0 (18.23% -24.86% 9 (10.23% -45.10%) and C18:2n-6 (3.50% -16.94%)
fatty acids [32]. In the study of Wen et al., the ratio of total
polyunsaturated n-3 and n-6 PUFA (43.86%) to phospholipid; the proportion of
total monounsaturated fatty acids (61.39%) was found to be high in neutral
lipid. Another important study on this subject was carried out by Hayashi and
Takagi [33]. In this study, researchers found that saturated fatty acids such
as myristic, palmitic and stearic acid taken via food and unsaturated fatty
acids such as oleic, linoleic and linolenic acid were stored directly in fish
oils and that the seasonal variation of these fatty acids was due to the phytoplankton,
zooplankton .In the present study titled "Fatty acid and lipid composition
of Water Mites (Acari, Hydracnidia) species by GC-MS", fatty acid ratios
in the waters (Eylais setosa, Hydrodroma despiciens and Hydrachna processifera)
are evaluated as saturated, monounsaturated and polyunsaturated in Tables 1-3. In this study, oil acid analysis was carried out by gas
chromatography (GC-MS) in water mites (Acari, Hydracnidia) species Hydrachna
processifera, Eylais setosa and Hydrodroma despiciens collected from Karamik
Lake. The results obtained were evaluated as saturated monounsaturated and
polyunsaturated fatty acids. The data were statistically evaluated and included
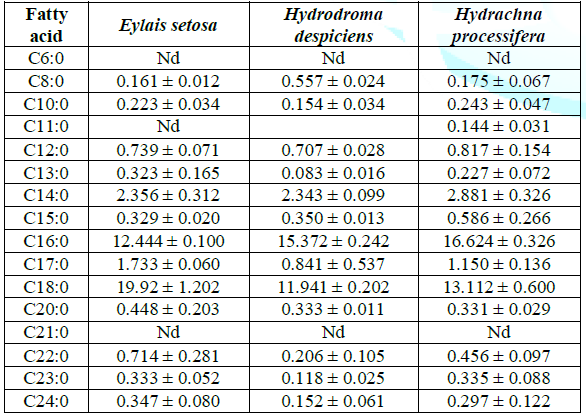
with standard deviations in Table 1. Results of saturated fatty acids: From Table 1, it is seen
that palmitic (C16:0) and stearic acid (C18:0) of saturated fatty acids are
found in high proportion in three of the water mites samples studied. According
to these numerical values, there is a remarkable difference between species in
terms of palmitic and stearic acid. Table
1: Average saturated fatty acid compositions (%) of water mites species in Lake
Karamık. The ratio of total saturated fatty acid was found 40.072%
for Eylais setosa, while Hydrodroma despiciens 33.299% and Hydrachna processifera
37.235% were found in other species. There was a significant difference in SFA
ratios between species. The change in total SFA rates is shown in Table 1 and
Figure 1. The ratio of stearic acid was found to be 11.941% in the
lowest Hydrodroma despiciens and 19.924% in the highest Eylais setosa. There
was no significant difference found between Hydrodroma despiciens and Hydrachna
processifera when compared to the other two species in Eylais setosa in terms
of stearic acid ratio.The lowest percentage of palmitic acid was found in
Eylais setosa (12.444%) and the highest in Hydrachna processifera (16.624%).
There was a significant difference in palmitic acid ratio compared to the other
two species of Eylais setosa, but no difference was observed between Hydrodroma
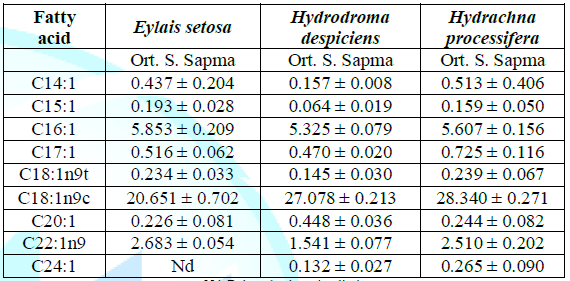
despiciens and Hydrachna processifera.Monounsaturated fatty acid results: The
changes in the monounsaturated fatty acid compounds in the samples are shown in
Table 2 and Figure 1. Table
2: Average monounsaturated fatty acid compositions (%) of water mites species
in Lake Karamık. Among the monounsaturated fatty acids, the most abundant
fatty acids are palmitoleic acid (C16:1), oleic acid (C18:1) and erucic acid
(C22:1). The total monounsaturated fatty acid content was found to be 38.638%
in Hydrachna processifera, 30.792% in Eylais setosa and 35.400% in Hydrodroma
despiciens. There was a significant difference in the MUFA ratios between the
species.Palmitoleic acid ratios was 5.325% in Hydrodroma despiciens and 5.853%
in Eylais setosa. In terms of palmitoleic acid ratios, there were no
significant differences detected between the species.The lowest oleic acid
content was found in Eylais setosa (20.651%) and the highest in Hydrachna
processifera (28.340%). In terms of oleic acid ratio, there was a significant
difference in comparison with the other two species of Eylais setosa, but no
difference was observed between Hydrodroma despiciens and Hydrachna
processifera. Erucic acid ratio was found to be 1.541% in Hydrodroma
despiciens and 2.683% in Eylais setosa. In terms of erucic acid ratios,
Hydrodroma despiciens showed a significant difference when compared to the
other two species, but no difference was observed between Eylais setosa and
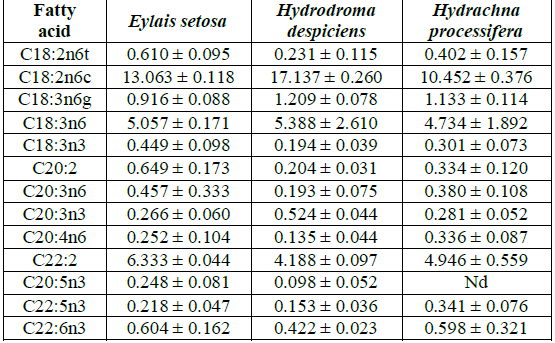
Hydrachna processifera.Polyunsaturated fatty acid results: The main polyunsaturated
ω3 fatty acids are linolenic acid (C18:3ω3), Eicosatrienoic acid (C20:3ω3),
Eicosapentaenoic acid (C20:5ω3) and Dokosahexaenoic acid (C22:6ω3). ω6 fatty
acids are Linoleic acid (C18:2ω6), γ-Linolenic acid (C18:3ω6), Eicosadienoic
acid (C20:2ω6) and Arachidonic acid (C20:4ω6).The total polyunsaturated fatty
acid ratio was found to be 30.076% in Hydrodroma despiciens, 29.122% in Eylais
setosa and 24.238% in Hydrachna processifera. There was a significant
difference in PUFA ratios between species. The variation of the total PUFA
ratios is shown in Table 3. Table
3: Average polyunsaturated fatty acid compositions (%) of water mites species
in Lake Karamik. In ω3 fatty acids, eicosatrienoic acid was found to be the
highest in Hydrodroma despiciens at 0.524% and the lowest in Eylais setosa at
0.266% and 0.281% in Hydrachna processifera. Hydrodroma despiciens in terms of
eicosatrienoic acid ratio was significantly different when compared to the
other two species, but no difference was observed between Eylais setosa and
Hydrachna processifera.Eicosapentaenoic acid in ω3 fatty acids was found to be
the highest in Eylais setosa at 0.248%, 0.098% in Hydrodroma despiciens and
below the detection limit in Hydrachna processifera. When compared to Eylais
setosa and Hydrodroma despiciens in terms of eicosapentaenoic acid ratios,
significant differences were observed.The highest concentration of
docosahexaenoic acid was found in Eylais setosa (0.604%) and the lowest level
was found in Hydrodroma despiciens (0.422%) and in Hydrachna processifera
(0.598%). Hydrodroma despiciens in terms of docosahexaenoic acid ratio was
significantly different when compared to the other two species, but no
difference was observed between Eylais setosa and Hydrachna processifera. Linolenic acid content was highest in Eylais setosa (0.449%)
and lowest in Hydrodroma despiciens (0.194%) and in Hydrachna processifera
(0.301%). Significant differences were observed between species in terms of
linolenic acid ratios.The highest ratio of arachidonic acid in ω 6 fatty acids
was 5.388% in Hydrodroma despiciens while the lowest was 4.734% in Hydrachna
processifera and 5.057% in Eylais setosa. There were no significant differences
found between the species in terms of arachidonic acid ratios.Among the ω6
fatty acids, C18:2ω6 was found to be the highest in Hydrodroma despiciens at
17.137% and the lowest in Hydrachna processifera at 10.452% and 13.063% in
Eylais setosa. There was a significant difference between species in terms of
C18:2ω6 ratios. Among ω6 fatty acids, the highest ratio of C22:2ω6 was
6.333% in Eylais setosa and the lowest was 4.188% in Hydrodroma despiciens and
4.946% in Hydrachna processifera. There was no significant difference between
Hydrodroma despiciens and Hydrachna processifera when compared to the other two
species of Eylais setosa in terms of C22:2ω6 ratio.Fatty acid compositions of
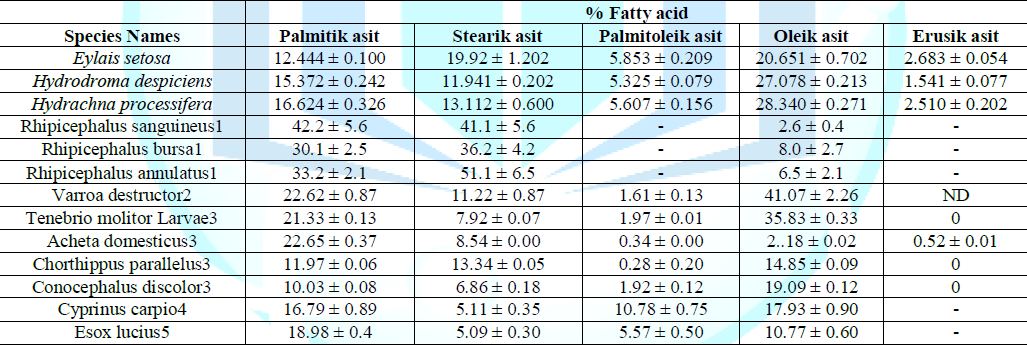
different species are given comparatively in Table 1. In terms of the fatty
acid (SFA, MUFA and PUFA) ratios found, the values of water mites species
(Hydrodroma despiciens,Hydrachna processifera, Rhipicephalus sanguineus) were
found to be close to each other. This was due to systematic closeness. Table 4
gives the fatty acid ratios in different species. Table
4: Comparison of fatty acid ratios in different species (%). It appears that the proportions of species close to each
other. It is also seen that the proportions of fish and water mites species
collected from the same locality are relatively similar. This can be explained
by the same locality and seasonal cycle. On the other hand, when we look at the
studies on other terrestrial parasites (Rhipicephalus sanguineus, Rhipicephalus
bursa, Rhipicephalus annulatus), it is seen that each fatty acid composition
compared to the water mites is quite variable for all three species given in
the table. The main reason for this is that each of these parasite species is
found as a host on a different animal. When the fatty acid compositions of each species were
examined in four different insect species belonging to insect class (Tenebrio
molitor Larvae, Acheta domesticus, Chorthippus parallelus, Conocephalus
discolor) where there is a great similarity in species belonging to the same
team species there is a big difference found between the ratios of both teams.
This is due to the fact that these three species belong to different teams of
the insect class and therefore differ in their systematic distance and
nutrition patterns. According to the table, another noteworthy situation is
that the fatty acid composition ratios in two different species of fish caught
in the same locality (Cyprinus carpio, Esox lucius) are surprisingly similar.
In this case, these observed results for these two species can also be
attributed to habitat similarity, seasonal characteristics and nutritional
factors. As a result, it can be said that each of the fatty acid
compositions is unique, and that systemic affinity, habitat and seasonal cycles
are also effective on these fatty acid compositions. All the results of this
study showed that this method can help to solve future taxonomic problems in
water mites. 1. Bulut S.
Fatty acid composition and 6/3 ratio of the pike (Esox lucius) muscleliving in
Eber Lake, Turkey (2010) Sci Res Essays 5: 3776-3780. 2. Bulut S,
Uysal K, Cemek M, Gok V, Kuş SF, et al. Nutritional evaluation of seasonal
changes in muscle fatty acid composition of common carp (Cyprinuscarpio) in
Karamık Lake, Turkey (2012) Int J Food Prop 15: 717-724. https://doi.org/10.1080/10942911003664891
3. Smit IM,
Cook DR. Ecology and classification of North American fresh water invertebrates
(1991) Thorp JH and Covich AP (eds.,) Academic, San Diego, CA, USA, pp:
523-592. 4. Di
Sabatino A, Smit H, Gerecke R, Goldschmidt T, Matsumoto N,et al. Global
diversity of water mites (Acari, Hydrachnidia; Arachnida) in fresh water (2008)
Hydrobiologia 595: 303-315. https://doi.org/10.1007/s10750-007-9025-1
5. Meyer E.
Der Entwick lungs zyklusvon Hydrodroma despiciens (O.F. Müller, 1776) (Acari.
Hydrodromidae) (1985) Arch Hydrobiol Suppl 66: 321-453 6. Smit H.
The water mite family Hygrobatidae Koch in Australia. The genera Aspidiobatella
Cook, Australorivacarus Viets, Gondwanabates Imamura and Rhynchaustrobates Cook
(Acari: Hydrachnidia) (2015) Zootaxa 4033: 567-583. https://doi.org/10.1007/s10493-013-9713-7
7. Tuzovskij
PV, Semenchenko KA. Morphology and taxonomy of deutonymphs of the genus
Unionicola Haldeman, 1842 (Acari, Hydrachnidia, Unionicolidae) in Russia (2015)
Zootaxa 3994: 69. https://doi.org/10.1007/s10886-005-7109-9 8. Boyacı
YO and Gülle P. New records of the water mite family Hydryphantidae (Acari:
Hydrachnidia) from Turkey, with the description of a new species (2014) Syst
Appl Acarol 19: 160-165. https://doi.org/10.11158/saa.19.2.6
9. Aşçı F,
Akkuş GU and Yaman İ. Determination of the ecological impact levels on
Hydrodromadespiciens (Müller 1776) (Acari, Hydrachnidia) which is a common
water mite in terms of heavy metal applications (2016) Pak J Zool 48: 345-348. 10. Aşçı F,
Bahadır M and Akkuş GU. Study on the impact of elements in water on the
diversity of water mites (Acari, Hydrachnidia) species (2015) Adv Bio Sci
Biotechnol 6: 259-264. https://doi.org/10.4236/abb.2015.64025
11. Onrat ST,
Asçi F and Ozkan M. A cytogenetics study of Hydrodroma despiciens (Müller,
1776(Acari: Hydrachnellae: Hydrodromidae) (2006) Genet Mol Res 30: 342-349. 12. Martin P,
Koester M, Schynawa L and Gergs R. First detection of prey DNA in Hygrobates
fluviatilis (Hydrachnidia, Acari): A new approach for determining predator-prey
relationships in water mites (2015) Exp Appl Acarol 67: 373-380.https://doi.org/10.1016/j.cbpa.2007.02.010
13. Dorda BA,
Valdecasas AG (2002) Traditional water mite fixatives and their compatibility
with later DNA studies. Exp Appl Acarol 34: 59-65.https://doi.org/10.1023/b:appa.0000044439.21180.ec
14. Bohonak
AJ, Smith BP and Thornton M. Distributional, morphological and genetic
consequences of dispersal for temporary pond water mites (2004) Fresh water
Biol 49: 170-180. https://doi.org/10.1111/j.1365-2426.2003.01177.x 15. Ernsting
BR, Edwards DD, Vidrine MF, Myers KS and Harmon CM. Phylogenetic relationships
among species of the subgenus Parasitatax (Acari: Unionicolidae: Unionicola)
based on DNA sequence of the mitochondrial cytochrome oxidase I gene (2006) Int
J Acarol 32: 195-202.https://doi.org/10.1080/01647950608684461
16. Więcek M,
Martin P and Lipinski A. Water mites as potential long-term bioindicators in
formerly drained and rewetted raised bogs (2013) Ecol Indic 34: 332-335.https://doi.org/10.1016/j.ecolind.2013.05.019 17. Yücecan S
and Baykan S. Food chemistry, food control and analysis (1981) Vocational and
Technical Education Books, Studies and Programming Department Publications,
Turkey, pp: 105-124. 18. Farkas T.
Adaptation of fatty acid composition to temperature a study on Carp
(Cyprinuscarpio L.) liver slices (1984) Comp Biochem Physiol 79: 531-535. 19. Belitz HD
and Grosch W. Food Chemistry (1999) Springer, Berlin, Heidelberg, Germany, pp:
152-236.https://doi.org/10.1007/978-3-662-07281-3
20. Karabulut
HA and Yandı İ.Su ürünlerindeki omega-3 yağ asitlerinin önemi ve sağlık üzerine
etkisi (2006) Ege Üniversitesi Su Ürünleri Dergisi 23: 339-342. 21. Aboshi T,
Shimizu N, Nakajima Y, Honda Y, Kuwahara Y, et al. Biosynthesis of linoleic
acid in Tyrophagus mites (Acarina: Acaridae) (2013) INS Bioch Mol Bio 43:
991-996. https://doi.org/10.1016/j.ibmb.2013.08.002
22. Peyou-Ndi
MM, Watts JL and Browse J. Identification and characterization of an animal Δ
12 fatty acid desaturase gene by heterologous expression in Saccharomyces
cerevisiae (2000) Arch Biochem Biophys 376: 399-408. 23. Takada W,
Sakata T, Shimano S, Enami Y, Mori N, et al. Scheloribatid mites as the source
of pumiliotoxins in dendrobatid frogs (2005) J Chem Ecol 31: 2403-2415. https://doi.org/10.1007/s10886-005-7109-9
24. Zhou X R,
Horne I, Damcevski K, Haritos V, Green A and Singh S. Isolation and functional
characterization of two independently‐evolved fatty acid Δ12‐desaturase genes
from insects (2008) Ins Mol Bio 1: 667-676. https://doi.org/10.1111/j.1365-2583.2008.00841.x
25. Raspotnig
G and Krisper G.Fatty acids as cuticular surface components in oribatid mites
(Acari: Oribatida), Ebermann, E. [Ed] Arthropod Biology: Contributions to
Morphology, Ecology and Systematics (1998) Biosyst Ecol Ser. 26. Shimshoni
J A, Erster O, Rot A, Cuneah O, Soback S and Shkap V. Cuticularfatty acidpro
file analysis of three Rhipi cephalu stick species (Acari: Ixodidae) (2013) Exp
Appl Acarol 61: 481-489. https://doi.org/10.1007/s10493-013-9713-7
27. Folch J,
Lees M and Sloane Stanley GH. Hayvan dokularından toplam lipitlerin izolasyonu
ve saflaştırılması için basit bir yöntem (1957) J Biol Chem 226: 497-509. 28. Paquot C.
Standard methods for the analysis of oils, fats and derivatives (1979) Pure
Appl Chem 51: 2503-2526. 29. AOCS.
Official methods and recommended practices of the american oil chemists society
(2nd edtn) (1972) Is Oil Chem Soc, USA. https://doi.org/10.1007/bf02582467 30. Murungi
LK, Kirwa H and Torto B. Differences in essential oil content of berries and
leaves of Solanum sarrachoides (Solanaceae) and the effects on oviposition of
the tomato spider mite (Tetranychusevansi) (2013) Ind Crop Prod 46: 73-79. https://doi.org/10.1016/j.indcrop.2013.01.022 31. Maazouzi
C, Masson G, Soledad Izquierdo M and Pihan JC. Fatty acid composition of the
amphipod Dikerogammarusvillosus: Feeding strategies and trophic links (2007)
Comp Biochem Physiol 147: 868-875.https://doi.org/10.1016/j.cbpa.2007.02.010 32. Wen X,
Chen L, Ku Y and Zhou K. Effect of feeding and lack of food on the growth,
gross biochemical and fatty acid composition of juvenile crab,
Eriocheirsinensis (2006) Aqua cult 252: 598-607. https://doi.org/10.1016/j.aquaculture.2005.07.027
33. Hayashi K
and Takagi T. Seasonal variations in lipids and, fatty acids of Japanese
anchovy, Engraulis japonica (1978) Full Faculty of Fisheries, Hokkaido
University, Japan 29: 38-47. Aşçi F, İnak M and Bulut S. Systematic evaluation of fatty acid profiles in Hydrachna processifera, Eylais setosa and (Acari, Hydrachnidia) species by GC-MS method (2019) Biochem and Modern Appli 2: 46-50. Ferruh Aşçi, Department of Molecular Biology and Genetics, Afyon Kocatepe University, Afyonkarahisar, Turkey, Fax no: +902722281235, Email: f_asci@aku.edu.tr Water mite, Acari, Hydrachnidia, Fatty acid composition
Systematic evaluation of fatty acid profiles in hydrachna processifera, eylais setosa and hydrodroma despiciens (acari, hydrachnidia) species by gc-ms method
Abstract
Full-Text
Introduction
Some notable of these
are as follows:
Materials and Method
The present study was carried out on water mites Hydrachna processifera,
Eylais setosa and Hydrodroma despiciens species (Acari; Hydracnidia) collected
from Karamik Lake within the boundaries of Afyonkarahisar province. Species
determinations were made under the microscope in the laboratory environment.Gas chromatographic
conditions were follows:
Results and
Discussion
Saturated and
unsaturated fatty acid results
References
Citation:
*Corresponding author:
Keywords