Review Article :
Amira Mohammed Ali and Amin Omar Hendawy 10-hydroxy-trans-2-decenoic acid (10H2DA), also
known as royal jelly acid, is the main lipid component of RJ. It possesses
anti-tumor, neurogenic, anti-inflammatory, antioxidant, bactericidal,
nematocidal, and estrogen-like properties. A limited number of studies demonstrate
the potentials of its main fatty acid, 10-H2DA, for alleviating anxiety and
depressive-like behaviors as well as for enhancing neuronal functioning.
However, the exact mechanism through which 10-H2DA produces its effect is not
well-understood. This mini review gives examples of how 10H2DA might positively
contribute to the treatment of psychiatric and neurological disorders. In
addition, it surveys the available knowledge about the molecular mechanism
through which it regulates transcriptional processes and gene expression in the
brain. Stress,
a main contributor to various psychiatric and neurodegenerative
disorders, leads to cellular death through destruction of biomolecules such
as DNA. Stress-induced detrimental effects are mediated by oxidative and
nitrosative stress i.e., increased lipid peroxidation and production of free
radicals such as Reactive Oxygen Species (ROS) and Nitric Oxide Species (NOS) [1].
Free radicals stimulate neuroinflammation via activation of inflammatory
pathways, which involve stimulation of inflammasome - a key component that
activates IL-1β and IL-18 [2]. On the other side, activation of inflammatory
pathways results in further internal NO cellular production [3]. In
addition, stress suppresses hippocampal neurogenesis;
significantly smaller hippocampi of depressed patients compared with healthy
individuals indicate sub-optimal neurogenesis [4]. Research documents that
stress-related mood disorders (e.g., depression and anxiety) are associated
with impaired function of hippocampal Brain-Derived Neurotrophic Factor (BDNF),
a member of the neurotrophin family of neurotrophic factors that plays roles in
neurogenesis as well as preservation of neuronal function and plasticity during
development and adulthood [5]. Unfortunately,
the mechanism through which psychiatric treatments such as antidepressants work
results in byproducts that stimulate oxidative stress, activate inflammatory
pathways such as nuclear factor-κB (NF-κB), alter neuroplasticity and
neurotrophins, impair mitochondrial function and neuronal bioenergetics;
and cause DNA damage, apoptosis, and cell death. Hence, the available
psychiatric drugs fail to produce the intended therapeutic goals and further
result in a trail of noxious adverse effects and serious complications [6,7].
Therefore, failure of existing treatments necessitates the search for safer
natural alternatives. Royal
Jelly (RJ), a creamy secretion of the hypopharyngeal and mandibular glands of
bee workers, is the main food of queen bees. Because it is a rich mixture of
proteins, lipids, sugars, vitamins, and minerals, RJ is considered a target
nutraceutical, and it has been used to treat various health problems [8].
Lipids constitute between 7 and 18% of the content of RJ [9], and they are
composed mainly of short hydroxy fatty acids with 8-12 carbon atoms in the
chain and dicarboxylic acids. 10-hydroxy-trans-2-decenoic acid (10H2DA), known
as royal jelly acid or queen bee acid, is a unique medium-chain unsaturated
fatty acid that exists only in RJ [8,10,11]. 10H2DA
constitutes the vast majority of the RJ lipid content (0.75 to 3.39%), and it
represents one of the main bioactive components of RJ [9]. A limited number of
studies demonstrate the potential of 10H2DA for enhancing neuronal functioning.
This mini review summarizes the available experimental and cell culture studies
that address the effects of 10H2DA on psychiatric and neurological disorders
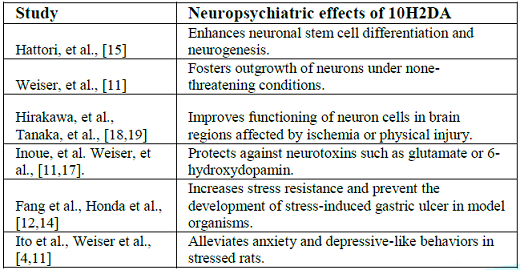
as well as the underlying molecular mechanisms. Few
experimental studies were conducted to test the effect of 10H2DA on
neuropsychiatric disorders, and it seems that 10H2DA may counteract
stress-induced destructive effects (Table
1). For
example, 10H2DA prevented the development of stress-induced gastric ulcer in
rats [12]. Needless to say, the gastrointestinal protective events of 10H2DA
against stress may contribute to protection of the brain given that enteric
neurons of the gut represent a main site for the production of
neurotransmitters such as serotonin and that the gut-brain axis plays a major
role in the development of neuropsychiatric disorders
[7,13]. More, 10H2DA was found to enhance longevity and increase stress resistance
of Caenorhabditis elegans against
thermal, irradiation, and oxidative stress [14]. Evidence
documents that 10H2DA mimics the effect of brain-derived nerotrophic factor in
progenitor cells (PC12) and stimulates neurogenesis by enhancing neuronal
differentiation from progenitor
nerve cells (PC12) [15]. Simultaneous intraperitoneal administration of
10H2DA (once a day for 3 weeks) in rats exposed to stress resulted in
improvement of depressive-like behaviors [4]. Similarly,
long term oral supplementation of 10H2DA (12–24 mg/kg/day for 5 months) to aging
rats significantly relieved anxiety-related behaviors as it increased the
amount of time spent in the open arm of the elevated plus maze. In the same
study, intraperitoneal injection of 10H2DA (100–500 g/kg/day for 3 weeks)
significantly reduced anxiety-like and depressive-like behavior
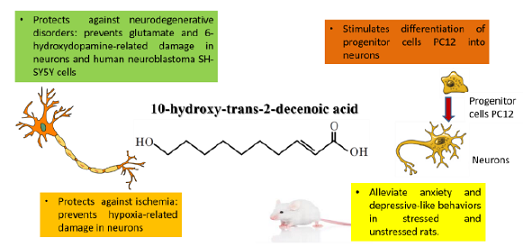
in stressed young mice (49–70 days old). Authors attributed this effect to the
estrogenic activity of 10H2DA as well as to its neurotrophic activity—inducing
the expression of BDNF through activation of Extracellular Signal-Regulated
Kinase (ERK) signaling [11] (Figure 1). Trans-2-decenoic
acid ethyl ester (DAEE) and 4-Hydroperoxy-2-decenoic acid ethyl ester
(HPO-DAEE) are 10H2DA-related esters that represent artificially synthesized
derivatives of RJ lipids [16]. These derivatives demonstrate neuroprotective
effects. Both DAEE and HPO-DAEE significantly prevented cell death in human
neuroblastoma SH-SY5Y cells that were challenged with 6-hydroxydopamine
(6-OHDA) as a model of Parkinsons
disease [17]. Intraperitoneal administration
of DAEE (100 and 150 μg/kg body weight) after hemisection of the spinal cord or
unilateral permanent middle cerebral artery occlusion in rats significantly
decreased the lesion size, prevented neurological deficits such as motor
paralysis, and improved functional recovery of the remaining intact neurons in
damaged regions. These effects were attributed to DAEE activation of ERK1/2,
and increased expression of bcl-2 and BDNF in the injury site [18,19]. Figure 2 summarizes the
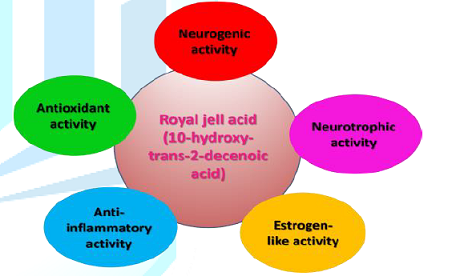
most probable mechanisms through 10H2DA might affect the Central Nervous System
(CNS) in order to produce therapeutic effects, namely: neurogenic and
neurotrophic activity, estrogen-like activity, antioxidant activity, and
anti-inflammatory activity. The following section explores these activities in
detail. A
main mechanism through which 10H2DA exerts its therapeutic activity involves
enhancement of neurogenesis and neuronal functions. It is reported that 10H2DA
increases neurogenesis, but decreases glial generation, of cultured neural
stem/progenitor cells. The exact mechanism involved is not clear; however, the
authors suggested that 10H2DA mimics the effect of BDNF [10]. Neurons treated
with 10H2DA significantly grew larger and made more interconnections with one
another. It also prevented cellular death and increased properly polarized
mitochondria in neurons challenged with glutamate and hypoxia as models of
age-related neurodegeneration
and stroke compared with untreated controls [11]. In
addition, treatment of neuron
cell cultures of embryonic rats with DAEE resulted in increase of
synapse-specific proteins [20]. The
neurogenesis, neurite outgrowth-promoting activity, synapse formation-
promoting activity, and neuroprotective properties of 10H2DA and its
derivatives are attributed to their neurotrophin-like effect fostering the
expression of bcl-2, BDNF, neurotrophin-3, and synapse-specific proteins (e.g.,
synaptophysin, synapsin-1, and syntaxin) which is mediated by the
phosphorylation of extracellular signal-regulated kinase 1 or 2 (ERK1/2), Mitogen
Activated Protein Kinase (MAPK), and cAMP Response Element-Binding Protein (CREB)
in neurons [18-20]. Among
the various fatty acids of RJ, 10H2DA, 10-hydroxydecanoic acid,
trans-2-decenoic acid, and 24-methylenecholesterol were found to affect
estrogen receptors and mediate estrogen signaling by modulating the activity of
Estrogen Receptors (ERs) ERα, ERβ [21,22]. Indeed, RJ fatty acids favorably
bind to ERα at the co-activator-binding site [21]. ERs regulate gene expression
and transcriptional processes through several mechanisms: 1) binding of
estrogen to receptors in the nucleus, then ERs dimerize and bind to Estrogen Response Elements
(EREs) located in the promoters of target genes; 2) protein-protein
interactions with other DNA-binding transcription factors in the nucleus; and
3) altering functions of cytoplasmic proteins through mediation of nongenomic
actions of estrogen leading to regulation of gene expression [23]. This effect
is likely to contribute to the neurogenic effects of these fatty acids since
estrogen can modulate cell proliferation and the expression of genes associated
with brain function and body composition [22]. Counteracting
oxidative stress and enhancing antioxidant capacity represent another mechanism
through which RJ lipids and their derivative might work. HPO-DAEE, 10H2DA, and
two other main fatty acids that exist in RJ (10-hydroxydecanoic acid and
sebacic acid) stimulated the expression of Extracellular Superoxide
Dismutase (ECSOD) in THP-1 cells through enrichment of acetylated histone
H3 and H4 in the in the proximal promoter region of ECSOD whereas only HPO-DAEE
activated the phosphorylation of ERK [16]. Nonetheless, reports on the
antioxidant effects of RJ fatty acids in the CNS are controversial. 10H2DA,
10-hydroxydecanoic acid, and sebacic acid failed to counteract 6-OHDA-induced
cellular death in in human neuroblastoma SH-SY5Y cell cultures. However, both
DAEE and HPO-DAEE significantly prevented cell death and stimulated the
production of antioxidant enzymes such as heme oxygenase-1 (HO-1). HPO-DAEE
demonstrated stronger antioxidant effect compared with DAEE [17]. On the
contrary, 10H2DA (300 mM) caused acute massive drop of the mitochondrial
electrical potential along with a simultaneous reduction of the NAD(P)H signal
in astrocytes, but not in neurons. Despite blocking of the respiratory chain,
intracellular ATP levels were unchanged secondary to a compensatory mechanism
that involved stimulation of glycolysis and augmentation of lactate formation
by 49.6% [24]. One
possible mechanism through which 10H2DA functions is by counteracting
inflammation indicated in a number of studies by NO production, which occurs as
a result of activation of NF-κB that follows TNF-α production; the latter was
stimulated by cellular challenge (e.g., with Lipopolysaccharide (LPS) and
interferon (IFN)-γ) [3,25]. Nevertheless,
reports on the molecular events involved in the anti-inflammatory effects of
10H2DA are inconsistent. 10H2DA, 10-hydroxydecanoic acid, and sebacic acid
inhibited the release of the major inflammatory-mediators, NO, and
interleukin-10 in a dose-dependent fashion, whereas only sebacic acid inhibited
TNF-𝛼 production in LPS-challenged
RAW264.7 macrophages. These effects were mediated by regulation of proteins
involved in MAPK and NF-𝜅B signaling pathways [26]. Similarly,
10H2DA inhibited the production of NO and TNF-α induced by LPS, IFN-β, and
IFN-γ challenge through modulation of cellular responses to these components
via suppression of IFN-β-induced NF-κB signaling and IFN-γ-mediated induction
of interferon
regulatory factor (IRF)-8. On the contrary, 10H2DA had no effect on
LPS-stimulated IFN-β production, IFN regulatory factor-1 induction and
IFN-stimulated response element activation, which are required for NOS
induction [3,25]. Moreover,
treating rheumatoid
arthritis synovial fibroblasts with 10H2DA (0.5 mM, 1 mM, and 2 mM) for 2 h
followed by stimulation with TNF-α (10 ng/ml) for 2 h had no effect on ERK
activity, NF-κB DNA-binding activity and IκBα degradation. Meanwhile, 10H2DA
caused blocking of p38 kinase and c-Jun N-terminal kinase–activator protein-1
(AP-1) signaling pathways [27]. This
review indicates that 10H2DA possesses neurotrophic, estrogen-like,
antioxidant, and anti-inflammatory properties, which suggest that 10H2DA might
be a promising avenue for ameliorating symptoms
of depression and anxiety as well as for improving neuronal survival and
functional recovery after CNS injury. Synthesized derivatives of 10H2DA (DAEE
and HPO-DAEE) demonstrate superior antioxidant and neurotrophic properties
compared with 10H2DA and other RJ fatty acids [16,17]. They even uphold an
extra merit of functioning at better conditions (e.g., neutrality) whereas
10H2DA produces its effects under certain conditions (e.g., it prevents the
development of transplantable AKR leukemia when the pH is below 5.6) [28]. Thus,
it might be crucial to compare the biological activities of 10H2DA, its
derivatives, and other RJ fatty acids, which seemed to produce effects similar
to 10H2DA. Remarkably, most of the reported estrogenic, antioxidant, and anti-inflammatory
properties of 10H2DA were obtained from cell lines other than neurons and
glial cells. Even more, the antioxidant effect of 10H2DA on neurons was
suboptimal while application of 10H2DA to astrocytes inhibited the metabolism
of mitochondria which could be a toxic effect given that 10H2DA suppressed
gliogenesis from neural stem cells. Therefore, sound evaluation of the
properties of 10H2DA and its derivatives in neurons and microglia is necessary.
Experimental studies should address issues related to dosing as well as proper
route and duration of administration before it could be used in clinical
trials. Special attention should be given to confounders of treatment as well. 1.
Ali
AM and Kunugi H. Bee honey protects astrocytes against oxidative stress: A
preliminary in vitro investigation (2019) Neuropsychopharmacol Rep 1-3. https://doi.org/10.1002/npr2.12079 2.
Czarny
P, Wigner P, Galecki P and Sliwinski T. The interplay between inflammation,
oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in
depression (2018) Prog
Neuropsychopharmacol Biol Psychiatry 80: 309-321. https://doi.org/10.1016/j.pnpbp.2017.06.036 3.
Takahashi
K, Sugiyama T, Tokoro S, Neri P, and Mori H. Inhibition of
interferon-gamma-induced nitric oxide production by 10-hydroxy-trans-2-decenoic
acid through inhibition of interferon regulatory factor-8 induction (2012) Cell
Immunol 273: 73-78. https://doi.org/10.1016/j.cellimm.2011.11.004 4.
Ito
S, Nitta Y, Fukumitsu H, Soumiya H, Ikeno K, et al. Antidepressant-like
activity of 10-hydroxy-trans-2-decenoic acid, a unique unsaturated fatty acid
of royal jelly, in stress-inducible depression-like mouse model (2012)
Evidence-based Complementary and Alternative Medicine: eCAM 139140. http://dx.doi.org/10.1155/2012/139140 5.
Toratani A, Soga H, Fukumitsu H, Soumiya H, Furukawa Y, et al.
Caffeic acid phenethyl ester ameliorates depression- and anxiety-like behaviors
of mice exposed to chronic mild stress (2014) J Neurophysiol Neurol Disord 1: 1-8. 6.
Ali AM, Hassan AA and Hendawy AO. The adverse effects of
antidepressant medication treatments on the offspring of women with perinatal
depression (2019) Scientific Journal of Research and Review 1. 7.
Ali
AM and Hendawy AO. So, antidepressant drugs have serious adverse effects, but
what are the alternatives? (2018b) Nov Appro Drug Des Dev 4: 555636. http://dx.doi.org/10.19080/NAPDD.2018.04.555636 8.
Kunugi H and Ali AM. Royal jelly and its components promote
healthy aging and longevity: from animal models to humans (2019) Int J Mol Sci
20: 4662. https://doi.org/10.3390/ijms20194662 9.
Kocot
J, Kielczykowska M, Luchowska-Kocot D, Kurzepa J, and Musik I. Antioxidant potential
of propolis, bee pollen, and royal jelly: possible medical application (2018)
Oxid Med Cell Longev 7074209. http://dx.doi.org/10.1155/2018/7074209 10. Hattori N, Nomoto
H, Fukumitsu H, Mishima S andFurukawa S. Royal jelly and its unique fatty acid,
10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural
stem/progenitor cells in vitro (2007b) Biomed Res 28: 261-266. http://dx.doi.org/10.2220/biomedres.28.261 11. Weiser MJ,
Grimshaw V, Wynalda KM, Mohajeri MH, and Butt CM. Long-term administration of
Queen Bee Acid (QBA) to rodents reduces anxiety-like behavior, promotes
neuronal health and improves body composition (2017) Nutrients 10. http://dx.doi.org/10.3390/nu10010013 12.
Fang
E, Zhou H, Xu H and Xing M. Antiulcer effects of 10-hydroxy-2-decenoic in rats
(1994) Chinese Pharmacological Bulletin 10. 13. Ali
AM and Hendawy AO. Bee honey as a potentially effective treatment for
depression: a review of clinical and preclinical findings (2018a) JOJ Nurse
Health Care 9: 555764. 14.
Honda
Y, Araki Y, Hata T, Ichihara K, Ito M, et al. 10-Hydroxy-2-decenoic acid, the
major lipid component of royal jelly, extends the lifespan of caenorhabditis elegans through dietary
restriction and target of rapamycin signaling (2015) J Aging Research 425261-425261. http://dx.doi.org/10.1155/2015/425261 15.
Hattori
N, Nomoto H, Fukumitsu H, Mishima S and Furukawa S. Royal jelly-induced neurite
outgrowth from rat pheochromocytoma PC12 cells requires integrin signal
independent of activation of extracellular signalregulated kinases (2007a)
Biomed Res 28: 139-146. http://dx.doi.org/10.2220/biomedres.28.139 16. Makino
J, Ogasawara R, Kamiya T, Hara H, Mitsugi Y, et al. Royal jelly constituents
increase the expression of extracellular superoxide dismutase through histone
acetylation in monocytic THP-1 cells (2016) J Nat Prod 79: 1137-1143. http://dx.doi.org/10.1021/acs.jnatprod.6b00037 17. Inoue
Y, Hara H, Mitsugi Y, Yamaguchi E, Kamiya T, et al. 4-Hydroperoxy-2-decenoic acid ethyl ester protects against
6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2α-ATF4
pathways (2018) Neurochemistry International 112: 288-296. https://doi.org/10.1016/j.neuint.2017.08.011 18.
Hirakawa
A, Shimizu K, Fukumitsu H, Soumiya H, Iinuma M, et al. 2-Decenoic acid ethyl
ester, a derivative of unsaturated medium-chain fatty acids, facilitates
functional recovery of locomotor activity after spinal cord injury (2010)
Neuroscience, 171: 1377-1385. https://doi.org/10.1016/j.neuroscience.2010.10.004 19.
Tanaka
Y, Fukumitsu H, Soumiya H, Yoshimura S, Iwama T, et al. 2-decenoic acid ethyl
ester, a compound that elicits neurotrophin-like intracellular signals,
facilitating functional recovery from cerebral infarction in mice (2012) Int J
Mol Sci 13: 4968-4981. https://doi.org/10.3390/ijms13044968 20.
Makino
A, Iinuma M, Fukumitsu H, Soumiya H, Furukawa Y, et al. 2-Decenoic acid ethyl
ester possesses neurotrophin-like activities to facilitate intracellular
signals and increase synapse-specific proteins in neurons cultured from
embryonic rat brain (2010) Biomed Res 31: 379-386. https://doi.org/10.2220/biomedres.31.379 21.
Moutsatsou
P, Papoutsi Z, Kassi E, Heldring N, Zhao C, et al. Fatty acids derived from
royal jelly are modulators of estrogen receptor functions (2010) PLoS One 5: e15594-e15594.
https://doi.org/10.1371/journal.pone.0015594 22.
Suzuki
KM, Isohama Y, Maruyama H, Yamada Y, Narita Y, et al. Estrogenic activities of
fatty acids and a sterol isolated from royal jelly (2006) eCAM 5: 295-302.http://dx.doi.org/10.1093/ecam/nem036 23.
Bjornstrom
L and Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of
genomic and nongenomic actions on target genes (2005) Mol Endocrinol 19:
833-842. http://dx.doi.org/10.1210/me.2004-0486 24.
Thevenet
J, Marchi UD, Domingo JS, Christinat N, Bultot L, et al. Medium-chain fatty
acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte–neuron
lactate and ketone body shuttle systems (2016) The FASEB Journal 30: 1913-1926.
http://dx.doi.org/10.1096/fj.201500182 25.
Sugiyama
T, Takahashi K, Kuzumaki A, Tokoro S, Neri P, et al. Inhibitory mechanism of
10-hydroxy-trans-2-decenoic acid (royal jelly acid) against lipopolysaccharide-
and interferon-beta-induced nitric oxide production (2013) Inflammation 36:
372-378. http://dx.doi.org/10.1007/s10753-012-9556-0 26.
Chen
YF, Wang K, Zhang YZ, Zheng YF and Hu FL. In Vitro anti-inflammatory effects of
three fatty acids from royal jelly (2016) Mediators of inflammation
3583684-3583684. http://dx.doi.org/10.1155/2016/3583684 27. Yang
XY, Yang DS, Wei Z, Wang JM, Li CY, et al. 10-Hydroxy-2-decenoic acid from
Royal jelly: A potential medicine for RA (2010) J Ethnopharmacology 128:
314-321. https://doi.org/10.1016/j.jep.2010.01.055 28.
Townsend
GF, Brown WH, Felauer EE and Hazlett B. Studies on the in vitro antitumor activity
of fatty acids: IV. The esters of acids closely related to 10-hydroxy-
2-decenoic acid from royal jelly against transplantable mouse leukemia (1961)
Canadian J Biochem Physiol 39: 1765-1770. https://doi.org/10.1139/o61-195 10-hydroxy-trans-2-decenoic acid, Royal jelly, Complementary
therapy, Bioactive food, Neuropsychiatric disorders.Royal Jelly Acid, 10-Hydroxy-Trans-2-Decenoic Acid, for Psychiatric and Neurological Disorders: How helpful could it be?!
Abstract
Full-Text
Introduction
Evidence
from Experimental Studies
Possible Mechanisms Underlying the Action of 10H2DA
Neurogenic
and Neurotrophic Activity
Estrogen-Like
Activity
Antioxidant
Activity
Anti-Inflammatory
Activity
Conclusion
References
*Corresponding author:
Citation:
Keywords